-–убрики
- уклы (260)
- ћ€гкие игрушки (16)
- ¬ал€ние (10)
- Ѕижутери€ (211)
- Ёпоксидна€ смола (35)
- ƒекупаж (361)
- Ћепка (173)
- ѕапье-маше (14)
- Ўитье (418)
- Ўл€пки и головные уборы (176)
- —умки (60)
- Ћампы, люстры, светильники (61)
- ћебель (104)
- улинари€ (58)
- јвторска€ бумага (39)
- Ўкатулки, коробочки (92)
- ћыловарение (86)
- ¬еера (42)
- ÷веты (144)
- анзаши (43)
- «онтики (7)
- ћои работы (14)
- «доровье и красота (84)
- “рофическа€ €зва и раны (12)
- ѕлетение и плетение из газет (51)
- Ѕутылки декоративные (10)
- ƒекор разное (102)
- ѕодарки к праздникам (40)
- ƒеревь€ декоративные (26)
- оты (4)
- “орты (196)
- “рафареты и узоры (101)
- ¬€зание (263)
- “енерифе (26)
- ¬ышивка (212)
- –ушник (12)
- –исование (29)
- ‘отошоп (81)
- Ѕатик (2)
- —утажна€ техника (54)
- —вадьба (93)
- »стори€ костюма (40)
- ¬интаж (5)
- ћех (12)
- ƒл€ работы (9)
- Ќовый год и –ождество (62)
- —троительство и ремонт (1)
- ¬идео (19)
- Ўторы и гардины (21)
- –елиги€ (12)
- ƒети (8)
- ќткрытки (2)
- –емонт (10)
- ƒомики (5)
- ÷вет и цветотипы (13)
- ћаникюр, педикюр (11)
- ”мные мысли (1)
- ќбложки и софтбуки (2)
- —тиль одежды (2)
- ѕсихологи€ (1)
-ѕоиск по дневнику
-ѕодписка по e-mail
-»нтересы
-ѕосто€нные читатели
-—татистика
¬ложенные рубрики: “рофическа€ €зва и раны(12)
ƒругие рубрики в этом дневнике: Ўторы и гардины(21), Ўл€пки и головные уборы(176), Ўкатулки, коробочки(92), Ўитье(418), ÷веты(144), ÷вет и цветотипы(13), ‘отошоп(81), ”мные мысли(1), “рафареты и узоры(101), “орты(196), —утажна€ техника(54), —умки(60), —троительство и ремонт(1), —тиль одежды(2), —вадьба(93), –исование(29), –емонт(10), –елиги€(12), ѕсихологи€(1), ѕодарки к праздникам(40), ѕлетение и плетение из газет(51), ќткрытки(2), ќбложки и софтбуки(2), Ќовый год и –ождество(62), ћыловарение(86), ћои работы(14), ћех(12), ћебель(104), ћаникюр, педикюр(11), Ћепка(173), Ћампы, люстры, светильники(61), улинари€(58), уклы(260), оты(4), »стори€ костюма(40), «онтики(7), ƒомики(5), ƒл€ работы(9), ƒети(8), ƒеревь€ декоративные(26), ƒекупаж(361), ƒекор разное(102), ¬€зание(263), ¬ышивка(212), ¬интаж(5), ¬идео(19), ¬еера(42), ¬ал€ние(10), Ѕутылки декоративные(10), Ѕижутери€(211), Ѕатик(2), јвторска€ бумага(39)
Ћечение содой |
ƒневник |
—ода пищева€. Ћечение содой: сода дл€ горла, при молочнице, от изжоги и прыщей
http://www.inmoment.ru/beauty/health/soda.html

—ода пищева€
“акое вещество, как пищева€ сода, есть на кухне у всех. ≈Є ещЄ называют питьевой, и используют дл€ добавлени€ в выпечку, мыть€ посуды, ликвидации непри€тных запахов – например, очень хорошо мыть с содой холодильник. —ода – это щелочное соединение, которое химики называют гидрокарбонатом натри€, и большинству людей известно, что еЄ можно использовать, как лечебное средство при многих заболевани€х.
Ћечение содой
—ода от изжоги
—амое частое применение соды – дл€ сн€ти€ изжоги. —ода нейтрализует в желудке сол€ную кислоту, и оказывает быстрое действие, которое медики называют антацидным – изжога проходит; но расскажем об этом немного подробнее.
—ол€на€ кислота действительно нейтрализуетс€ содой, но при этом выдел€етс€ углекислота, оказывающа€ на слизистую оболочку желудка возбуждающее действие, и стимулирующа€ выделение гастрина – гормона, усиливающего секрецию желудочного сока, измен€ющего моторику желудка и кишечника, а также их тонус.
≈сли часто пользоватьс€ содой при изжоге (а так делают многие), то еЄ избыток начнЄт всасыватьс€ в кровь, и кислотно-щелочное равновесие нарушитс€ – начнЄтс€ ощелачивание крови. ѕоэтому лучше примен€ть специальные препараты, но ещЄ лучше – обратитьс€ к врачу, чтобы вы€снить причину изжоги – соду же (1 ч.л. на 1/3 стакана воды) стоит использовать только в качестве «скорой помощи».
—ода дл€ горла. ѕолоскание горла содой
ƒругой распространЄнный способ применени€ соды – при бол€х в горле, простуде, дл€ лечени€ инфекций слизистой оболочки рта, как отхаркивающее средство и т.д.
Ћечить содой горло очень просто: размешать в стакане воды ½ ч.л. соды, и полоскать горло этим раствором; повтор€ть каждые 3-4 часа, череду€ с другими средствами. —ода нейтрализует действие кислот, образующихс€ в горле при ангине, фарингите и других заболевани€х, и поэтому боль и воспаление уход€т.
—ода при простудах
—одова€ ингал€ци€ – тоже известное средство от простуды. ѕри насморке надо в небольшом чайнике довести до кипени€ стакан воды, добавить к ней 1 ч.л. соды, потом вз€ть трубочку из очень плотной бумаги, и надеть еЄ одним концом на носик чайника, а другой конец вставл€ть поочерЄдно то в одну, то в другую ноздрю – в общей сложности дышать таким паром около 15-20 минут.
ћожно использовать раствор соды, как капли в нос при насморке: кип€чЄной воды – 2 ч.л., соды – на кончике ножа; капать в нос по 2-3 раза в день.
ќтхождению в€зкой мокроты сода тоже помогает: надо пить натощак, 2 раза в день, по ½ стакана тЄплой воды, раствор€€ в ней щепотку соли и ½ ч.л. соды – однако долго так лечитьс€ тоже не следует.
—м€гчить кашель можно гор€чим молоком с содой. —оду (1 ч.л.) надо развести пр€мо в кип€щем молоке, слегка остудить и выпить на ночь.
√ор€чей смесью соды и картофельного пюре лечат бронхиты у детей и взрослых. артофель (несколько штук) надо сварить в кожуре, и сразу, в гор€чем виде, разм€ть его, добавив соду (3 ч.л.), потом быстро слепить 2 лепЄшки, завернуть их в полотенца и положить одну на грудь, а другую – на спину, между лопаток. ЋепЄшки должны быть гор€чими, но не обжигающими. ѕосле этого надо тепло укутать больного и уложить в постель. ЋепЄшки убрать, когда остынут, вытереть больного насухо и переодеть в сухую одежду.
—ода при молочнице
ћожно лечить содой и молочницу – заболевание, известное почти каждой женщине; мужчины и дети тоже могут заболеть, хот€ об этом мало кто знает. ћедики называют молочницу кандидозом, или кандидным вульвовагинитом – эта инфекци€ вызываетс€ дрожжевыми грибками рода андида.
ѕримерно в половине случаев сода помогает в лечении молочницы: раствор соды – это щелочь, а грибки в щелочной среде погибают – разрушаетс€ структура их клеток.
Ћечение молочницы содой имеет свои минусы и плюсы.
ѕлюсы: это дЄшево и сравнительно безопасно – если сравнивать с более агрессивными методами лечени€. ћинусов, возможно, больше. ѕрежде всего, сода помогает, как уже говорилось, только в 50% случаев; второй минус – спринцеватьс€ необходимо регул€рно и очень часто. ќдни врачи считают, что достаточно 2-х раз в день (1 ч.л. на литр кип€чЄной воды), а другие предлагают делать это каждый час, и не прекращать такое лечение в течение 2-х недель – иначе можно даже не начинать.
Ћечитьс€ содой можно, но сегодн€ по€вилось очень много различных препаратов дл€ лечени€ молочницы – стоит обратитьс€ к врачу, и он подберЄт то, что больше подходит – заниматьс€ самолечением вр€д ли следует. ¬ любом случае, обращатьс€ к специалистам придЄтс€: ведь молочница – не просто инфекци€, а грибки, в норме живущие в половых пут€х, и они вызывают заболевание только при благопри€тных услови€х дл€ его развити€. Ёто могут быть гормональные нарушени€ в организме; действие лекарств, в том числе гормонов и антибиотиков; сахарный диабет и заболевани€ щитовидной железы; ослабленный иммунитет и много других причин.
—ода от прыщей
¬ лечении такой проблемы, как прыщи, можно добитьс€ большего успеха, использу€ соду, к тому же эта процедура не настолько хлопотна€, как лечение молочницы.
¬ариантов лечени€ прыщей содой много.
Ќапример, можно растворить в стакане кип€тка сахар и соду (по 1 ч.л.), смочить полученным раствором ватный диск, и тщательно, но осторожно протереть им лицо, удел€€ больше внимани€ проблемным участкам; потом надо умыть лицо с хоз€йственным мылом, слегка тЄплой водой, и смазать кожу проблемных участков сливочным маслом. „ерез час снова умытьс€ тЄплой водой, но без мыла.
ћожно сразу использовать соду вместе с мылом – об этом способе многие отзываютс€, как о неплохом. ћыло надо натереть на мелкой тЄрке, распарить лицо – наклонитьс€ над паром, прикрывшись толстым полотенцем, и, слегка массиру€, протереть кожу ватным диском, насыпав на него мыло и соду; умытьс€ слегка тЄплой водой – достаточно делать это раз в неделю, а в остальные дни протирать лицо кубиками лимонного льда.
—ода в народной медицине
—оду примен€ют в лечебных цел€х при многих других заболевани€х. ѕри укусах насекомых – мошек и комаров, надо наложить на место укуса содовую кашицу на кусочке марли: зуд пройдЄт быстро, а покраснение постепенно исчезнет.
ћожно использовать соду дл€ профилактики кариеса: надо несколько раз в день полоскать рот еЄ раствором, или чистить содой зубы, как раньше их чистили зубным порошком. —ода не повреждает эмаль, зато нейтрализует образующиес€ во рту кислоты и полирует зубы, предотвраща€ их разрушение.
ќт непри€тного запаха изо рта можно избавитьс€, прополоскав рот раствором пищевой соды с перекисью водорода. ¬ стакан с раствором перекиси (2-3%) добавл€ют соду (1 ст.л.) и прополаскивают рот. –азумеетс€, следует узнать причину запаха изо рта, а не маскировать его посто€нно содовыми полоскани€ми: возможно, запах вызван серьЄзным заболеванием, поэтому лучше пройти полное обследование.
ѕри ревматизме помогают ванны и компрессы с травами и содой. ƒл€ лечебной ванны надо заварить травы – ромашку, шалфей, душицу (по 1 ст.л.) крутым кип€тком (1 л) и настаивать час. ѕотом процедить, добавить к настою 400 г соды и вылить раствор в ванну с водой – температура воды не должна быть выше 40°C, - добавить по нескольку капель эфирных масел лаванды и розмарина. ¬анну принимают на ночь, по 20-25 минут; после неЄ сразу ложатс€ в постель, обмотавшись шерст€ным платком.
ƒл€ того, чтобы сделать компресс, надо насыпать соду на свежий капустный лист, и приложить его к больному месту. —верху прикрыть плЄнкой и тЄплым платком, и лечь в постель – держать 2 часа. Ќа улицу сразу после компресса лучше не выходить. Ћечебные ванны с содой полезны при псориазе, сухих дерматитах и просто сухой коже тела. ¬ ванну добавл€ют 35 г соды, 20 г карбоната магнезии и 15 г пербората магни€ – сначала вода должна быть просто тЄплой, потом еЄ температуру постепенно увеличивают до 39°C; принимать ванну 15 минут.
ѕри отЄках ног раствор€ют 5 ст.л. соды в 5 л тЄплой воды, добавл€ют отвар м€ты с шалфеем (1 стакан), и принимают ванночку дл€ ног в течение 20-25 минут.
ѕоскольку сода решает многие косметические проблемы – с ней даже делают примочки новорождЄнным, если у них по€вл€ютс€ опрелости, - то еЄ можно использовать дл€ ухода за кожей и волосами. ƒл€ борьбы с жирной перхотью в кожу головы перед мытьЄм втирают раствор соды – 1 ч.л. соды на стакан воды.
—ода – довольно эффективное средство лечени€, и помогает облегчать и лечить многие заболевани€, но не стоит полагатьс€ на такой способ лечени€ в сложных случа€х: домашние средства часто нам помогают, но лучше всЄ же не рисковать, а обращатьс€ к специалистам.
јвтор: √атаулина √алина
—тать€ защищена законом об авторских и смежных правах. ѕри использовании и перепечатке материала активна€ ссылка на женский сайт inmoment.ru об€зательна!
|
Ћечение содой-2 |
ƒневник |
ќблегчит страдани€ сода
http://medic.ymka.ru/oblegchit_stradaniya_soda.php
ћетоды лечени€ народными средствами
ќЅЋ≈√„»“ —“–јƒјЌ»я —ќƒј
—реди лекарств, широко примен€емых в народной медицине, на одном из первых мест стоит обыкновенна€ питьева€ сода. Ётот белый порошок с солоноватым вкусом можно использовать при лечении самых различных недугов, особенно когда вы находитесь за городом и лишены возможности получить врачебную помощь. ¬начале напомним вкратце о достаточно широко известных рецептах, а затем перейдем к более редким.
• ќчень часто даже при небольшой простуде по€вл€етс€ кашель. „тобы см€гчить его, разведите 1 чайную ложку соды в стакане кип€щего молока и выпейте на ночь. ѕри необходимости повторите.
• ѕри воспалительных заболевани€х верхних дыхательных путей, а также при остром и хроническом ларингите хорошо помогают ингал€ции с содовым раствором. Ќалейте в чайник 1 литр воды и добавьте 1 столовую ложку соды. огда вода закипит, наденьте на носик чайника бумажную трубочку, но только не из газеты, и подышите паром в течение 10-15 минут.
• ѕри ангинах, ларингитах, фарингитах, а также при зубной боли, стоматитах и особенно при флюсе (воспалении надкостницы) нужно 5-6 раз в день полоскать рот и горло раствором питьевой соды из расчета 1-2 чайные ложки порошка на 1 стакан теплой или гор€чей воды.
• ѕри насморке содовый раствор можно использовать как капли: немного - на кончике ножа - соды следует развести в двух чайных ложках теплой воды и закапывать в нос 2-3 раза в день.
• ¬ случае внезапного приступа сердцебиени€ рекомендуетс€ прин€ть 1/2 чайной ложки питьевой соды, чтобы выровн€ть пульс.
• „тобы избавитьс€ от мигрени, можно ежедневно за 30 минут до еды пить кип€ченую воду с содой из расчета 1/2 чайной ложки порошка на 1 стакан воды. ¬ первый день до обеда нужно выпить 1 стакан, на второй день - 2 стакана, по одному перед обедом и ужином и т.д., довед€ до 7 стаканов в день. «атем ежедневно снижать количество принимаемой смеси на 1 стакан. Ќа этом лечение закончить.
• ѕричиной возникновени€ головных болей часто бывают нарушени€ функций желудка. ¬ таких случа€х следует выпить стакан молока комнатной температуры с небольшим количеством соды.
• ƒл€ очищени€ кишечника можно примен€ть содовые клизмы из расчета 1 чайна€ ложка питьевой соды на 1 литр воды дл€ одной процедуры.
• ” женщин при попадании инфекции в мочевывод€щие пути по€вл€ютс€ частые позывы к мочеиспусканию, боль и жжение при нем. ѕри первых же таких симптомах рекомендуетс€ выпить содовый коктейль из расчета 1 чайна€ ложка порошка на стакан воды.
• ѕри остром геморрое помогают холодные примочки с 2-процентным раствором питьевой соды, которые нужно мен€ть через каждые 30 минут.
• ¬ случае панарици€, гнойного воспалени€ пальца, приготовьте крепкий содовый раствор, растворив 2 столовые ложки порошка в 0,5 литра гор€чей воды, опустите в него больной палец и подержите 15-20 минут. Ёту процедуру нужно проводить 3 раза в день.
• ѕрохладный раствор соды из расчета 1 чайна€ ложка на стакан воды уменьшит зуд от укуса комаров. Ётот же раствор можно использовать и дл€ отпугивани€ насекомых, несколько раз в день протира€ им кожу. роме того, им полезно протирать подмышечные впадины, поскольку сода нейтрализует кислую среду, в которой размножаютс€ бактерии, придающие поту непри€тный запах.
• ѕри крапивнице с обильной сыпью по всему телу рекомендуетс€ дважды в день принимать теплые содовые ванны, растворив 400 г порошка на каждую. ѕосле процедуры обтереть тело водкой или водой с уксусом, либо свежим томатным соком.
• — наступлением жаркой погоды у маленьких детей часто по€вл€етс€ потница - скопление небольших розовых прыщиков. ƒл€ избавлени€ от нее нужно несколько раз в день обрабатывать пораженные места тампоном, смоченным в содовом растворе из расчета 1 чайна€ ложка порошка на 1 стакан воды.
• ѕри жирной перхоти рекомендуетс€ перед мытьем головы развести 1 чайную ложку соды в стакане воды и втирать раствор в кожу.
• ƒл€ удалени€ мозолей на руках следует 2-3 раза в неделю держать их в течение 10 минут в ванночке с теплой содовой водой из расчета 1 чайна€ ложка порошка на 1 л, а затем вытереть насухо и потереть пемзой.
• „тобы избавитьс€ от повышенной потливости ног, нужно утром и вечером обмывать их раствором соды из расчета 1 чайна€ ложка на стакан воды. ј на ночь следует положить между пальцами ватки, смоченные этим же раствором. ≈сли будет чесатьс€ или даже саднить, потерпите.
• ƒл€ лечени€ грибковых поражений ног в 1 столовую ложку соды добавить немного воды комнатной температуры и натереть этой смесью пораженные места. «атем ополоснуть водой, дать высохнуть и присыпать крахмалом.
• „тобы сн€ть отечность и усталость ног, достаточно в течение 15 минут подержать их в ванне с содой из расчета 5 столовых ложек порошка на 10 л теплой воды.
• ѕри термических ожогах рекомендуетс€ промыть поврежденное место содовым раствором (чайна€ ложка на стакан воды) и приложить к нему марлевую салфетку, смоченную в этом растворе.
• ѕитьева€ сода помогает сн€ть похмельный синдром. ѕри легкой степени нужно прин€ть 3-4 г питьевой соды в первые 2-3 часа, при среднет€желой - до 6-8 г, а при т€желой - до 10 г в течение 12 часов.
|
Ћечение содой-3 |
ƒневник |
18 рецептов лечени€ питьевой содой
http://www.polezen.ru/health/recept/soda.php
ѕитьевой содой можно лечить всю семью от разных хворей. ќ ее лечебных свойствах рассказывает преподаватель расногорского медицинского училища из ѕодмосковь€ “ать€на Ћосото.
1. ¬кус соды, растворенной в молоке, знаком с детства каждому. » по сей день это лучшее средство дл€ см€гчени€ кашл€ - сода прекрасно разжижает мокроту. ¬рачи рекомендуют развести одну чайную ложку соды в кип€щем молоке и принимать на ночь.
2. “ем, кто не любит или не переносит молоко, при кашле помогут ингал€ции с содовым раствором - столова€ ложка на литр кип€щей воды.
3. Ќичто так хорошо не снимает боль в горле, как полоскание раствором питьевой соды - две чайные ложки на стакан теплой воды. ѕолоскать надо п€ть-шесть раз в день. —ода увлажн€ет слизистую оболочку горла, тем самым уменьша€ першение.
4. —правитьс€ с насморком поможет закапывание в нос содового раствора. ѕри обильных выделени€х советую делать промывани€ - закапайте в нос несколько пипеток раствора, а через минуту очистите его от слизи. ѕроцедуру надо повтор€ть два-три раза в день.
5. ѕри конъюнктивитах помогают многократные промывани€ глаз раствором соды. “олько помните, что одной ваткой можно пользоватьс€ только один раз.
6. акой €звенник не прибегал к соде, чтобы избавитьс€ от болей и изжоги? ќна нейтрализует избыток кислоты в желудке, и улучшение наступает в считанные минуты. ѕоэтому сода долгие годы была основным лекарством от €звенной болезни. ќднако частое ее применение дает обратный эффект: выделение кислоты увеличиваетс€. роме того, при взаимодействии кислоты с содой выдел€етс€ углекислый газ, который бомбардирует истонченную стенку желудка, что может привести к прободению €звы. ѕоэтому соду следует использовать только когда под рукой нет других лекарств.
7. —ода издавна примен€етс€ в медицине как антиаритмическое средство. ¬незапный приступ сердцебиени€ можно прекратить, прин€в половинку чайной ложки.
8. ѕомогает сода и при гипертонии: благодар€ усиленному выведению жидкости и солей из организма она снижает артериальное давление. ѕоловина чайной ложки, прин€та€ вместе с лекарствами, позвол€ет уменьшить их дозу.
9. —ода - очень эффективное средство против укачивани€ в транспорте. √лавное - не забыть вз€ть с собой в дорогу порошок.
10. ≈сли кто-то обожжетс€ кислотой, то ее можно моментально нейтрализовать содовым раствором.
11. —ода - средство первой помощи при т€желых травмах, большой кровопотере, отравлени€х, протекающих с многократной рвотой и поносом, длительной лихорадке с проливными потами. „тобы восполнить потерю жидкости, надо приготовить содово-солевой раствор. –ецепт прост: половину чайной ложки соды и чайную ложку соли развести в одном литре теплой кип€ченой воды. ƒавать по 1 столовой ложке каждые п€ть минут.
12. Ќе обойтись без соды и больным с панарицием - гнойным воспалением пальца. Ћечение начинайте, как только по€витс€ пульсирующа€ боль. ѕриготовьте крепкий содовый раствор: две столовые ложки соды на поллитра гор€чей воды. ќпустите туда палец и подержите минут двадцать. ƒелайте это три раза в день - и воспаление об€зательно рассосетс€.
13. ѕолоскание рта с содой хорошо снимает зубную боль. ќсобенно эффективно оно при флюсе (воспалении надкостницы). ѕриготовив гор€чий содовый раствор, полоскайте им рот 5 - 6 раз в день. »ногда это позвол€ет избежать хирургического лечени€.
14. —ода - отличное косметическое средство. —мешав ее с мыльной стружкой, протирайте этой смесью лицо два раза в неделю. ќна хорошо помогает при юношеских угр€х, очища€ кожу от омертвевших клеток и открыва€ поры лица.
15. —ода может заменить отбеливающие зубные пасты. ќбмакнув в нее ватку, потрите зубы, пока не удалите желтый налет. –езультат виден даже после одной такой чистки.
16. Ќе преп€тству€ выделению пота, сода нейтрализует его кислую среду. ј как известно, именно в ней бурно плод€тс€ бактерии, которые и придают поту непри€тный запах. ѕоэтому в летнее врем€ по утрам полезно протирать подмышечные впадины ваткой, смоченной в растворе соды - запаха не будет весь день.
17. —одовый раствор помогает избавитьс€ от последствий укусов насекомых. ≈сли несколько раз в день смазывать им место укуса, то жжение и зуд исчезнут. роме того, сода предотвращает попадание микробов в ранку.
18. ѕосле трудового дн€ сн€ть усталость и отечность ног помогут ножные ванны с содой: п€ть столовых ложек на дес€ть литров теплой воды. ѕ€тнадцать минут - и вы сможете танцевать до самого утра!
|
Ћечение содой-4 |
ƒневник |
ѕри болезни почек помогает сода
http://www.health-ua.org/news/5855.html

ƒоктора из ¬еликобритании считают, что нашли доступное и дешевое средство, которое может значительно облегчить страдани€ пациентов с почечной недостаточностью. Ёто чудесное лекарство – обыкновенна€ пищева€ сода. –абота почек ухудшаетс€ гораздо медленнее, если пациент принимает немного соды раз в день. –езультаты исследовани€ были опубликованы в журнале Journal of the American Society of Nephrology.
ƒоктора давно интересовались, каким образом использование в лечении пищевой соды может повли€ть на почки пациентов, страдающих от низкого уровн€ бикарбоната. Ёто состо€ние называют метаболическим ацидозом. »сследователи из оролевского √оспитал€ в Ћондоне привлекли к своей работе 134 пациента, дава€ им небольшую дозу соды каждый день нар€ду с обычными лекарствами. „ерез год работа их почек ухудшилась в гораздо меньшей степени, чем у тех больных, которых не лечили содой. ”ровень ухудшени€ работы их почек был не намного заметнее, чем при обычном процессе старени€. ќни также реже страдали от последней стадии почечной болезни, требующей диализа.
«“акое простое средство, как пищева€ сода, если его правильно использовать, может быть чрезвычайно эффективным. ќно очень полезно дл€ людей с почечной недостаточностью. ќднако доза соды должна тщательно подбиратьс€, и такое лечение следует проводить под надзором специалистов», - заключил руководитель исследовани€ профессор ренальной медицины из оролевского √оспитал€ в Ћондоне ћагди якуб.
|
Ћечение содой-5 |
ƒневник |
Improved Growth in Young Children with Severe Chronic Renal Insufficiency Who Use Specified Nutritional Therapy
»сточник http://jasn.asnjournals.org/content/12/11/2418.full
†Department of Pediatrics, Montefiore Medical Center/Albert Einstein College of Medicine, Bronx, New York
‡Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor, Michigan
§Department of Pediatrics, University of Alabama, Birmingham, Alabama.
Correspondence to Dr. Rulan Parekh, Division of Pediatric Nephrology, Park 327, 600 N Wolfe Street, Johns Hopkins Hospital, Baltimore, MD 21287-2535. Phone: 410-955-2467; Fax: 410-614-3680; E-mail: rsparekh@jhmi.edu
Abstract
Abstract. Growth in children with chronic renal failure caused by polyuric, salt-wasting diseases may be hampered if ongoing sodium and water losses are not corrected. Twenty-four children were treated with polyuric chronic renal insufficiency (CRI; creatinine clearance <65 ml/min per 1.73 m2) with low-caloric-density, high-volume, sodium-supplemented feedings. Subsequent growth was compared with that of children in two control groups: a national historic population control from the US Renal Data System database (n = 42), and a literature control (n = 12). Members of the three groups were 81 to 96% white, and 58 to 70% were boys. Obstructive uropathy and dysplasia were the cause of CRI in 92% of the treatment group, 75% of the literature control group, and 30% of the population control group. Treatment effect was assessed in a multivariate, retrospective analysis of the height standard deviation score (SDS), simultaneously controlling for the severity of disease by renal replacement therapy, primary cause of CRI, and initial height SDS. The change in SDS (ΔSDS) for height by regression analysis at 1 yr was significantly greater by +1.37 in the treatment group versus the population control (P = 0.017). The 2-yr height ΔSDS by regression analysis adjusted for creatinine clearance was significantly greater by +1.83 in the treatment group versus the literature control (P = 0.003). Nutritional support with sodium and water supplementation can maintain or improve the growth of children with polyuric, salt-wasting CRI. This inexpensive intervention may delay the need for renal replacement therapy, growth hormone treatment, or both in many of these children and may be used in any clinical setting.
Introduction
The long-term prognosis of children with early-onset chronic renal failure has improved dramatically in the last decade as a result of technologic and pharmacologic improvements in dialysis and transplantation, aggressive nutritional support, correction of metabolic defects, and effective treatment of renal osteodystrophy. Despite these advances, however, growth failure continues to be a significant obstacle affecting children with renal failure. The greatest height deficit occurs in the first year of life (1), especially in the first 4 mo of life (2). Known factors contributing to growth failure include anorexia, caloric deficits, hyposthenuria, salt wasting, anemia, metabolic acidosis, mineral depletion, renal osteodystrophy, and growth hormone resistance (1,3,4,5,6).
Renal replacement therapy (RRT), irrespective of modality (dialysis or transplantation), does not always result in the correction of height deficits (7,8,9). This is especially true in children with congenital polyuric forms of end-stage renal disease (ESRD), most notably renal dysplasia and obstructive uropathy, the two most common forms of childhood ESRD (10). Children with these forms of ESRD commonly have height deficits >2 SD below the mean, despite aggressive, conservative management. The resulting height deficit frequently cannot be overcome, despite supplemented feedings (11,12), peritoneal dialysis (11), or even transplantation, which results in catch-up growth in <50% of younger children (7,9,13). Strategies are therefore needed to minimize pretransplant growth retardation.
Children with polyuric, salt-wasting forms of chronic renal failure whose sodium and water losses are not corrected may experience significant growth retardation related to chronic intravascular volume depletion and a negative sodium balance. We hypothesized that sodium and water supplementation in the form of low-caloric-density, high-volume, sodium-supplemented feedings could normalize the internal milieu in such children and promote normal growth. Our aim in this study was to evaluate the growth response to such feedings and to determine whether this nutritional intervention would result in improved growth compared with previously reported results.
Materials and Methods
Specified Nutritional Therapy
The nutritional therapy we prescribed for the children consisted of an enteral formula diluted with water to a caloric density of 0.3 to 0.5 kcal/ml and supplemented with 2 to 4 mEq of sodium (as chloride, bicarbonate, or both) per 100 ml of formula (Appendix). Children who received this formula all had chronic renal failure, defined as calculated GFR ≤65 ml/min per 1.73 m2, at the time the specified feedings were initiated. Formulas used included Nepro, Suplena, Similac PM 60/40 (Ross Products Division, Abbott Laboratories, Columbus, OH), and SMA (Wyeth-Ayerst Laboratories, Philadelphia, PA). Feedings were prescribed in volumes of 180 to 240 ml/kg per 24 h (depending on urine output), which provided 100 to 160 kcal/kg per 24 h and approximately 2 to 2.5 g/kg protein per day. Initial caloric and protein intakes for the patients reported here were 120 kcal/kg per day and 2.0 to 2.5 g/kg per day. The corresponding recommended dietary allowances (RDA) for infants and toddlers <3 yr old are 98 to 108 kcal/kg per day and 1.2 to 2.2 g/kg protein per day (14).
Growth parameters, including initial length by infant stadiometer, standing height by stadiometer, and weight by standard balance were measured. Laboratory studies including electrolytes, acid base status, and intact parathyroid hormone level were performed on a regular basis (usually monthly). All patients received vitamin D therapy if they developed an elevation of the intact parathyroid hormone more than three times normal. A renal formulated multivitamin was prescribed (Iberet Abbott Laboratories, North Chicago, IL; Nephro- Vite, R&D Laboratories, Marina del Rey, CA; Nephrocaps, Fleming & Co., Fenton, MO) for all patients. No child received growth hormone.
Feeding volume and caloric intake were regularly adjusted on the basis of increases in height and weight. During regular monthly or bimonthly visits, fluid intake was increased according to gain in body weight, and calories were adjusted up or down depending on linear growth, head circumference, and albumin levels during the previous few months. The sodium was prescribed as milliequivalents per 100 ml of formula, and increases in formula volume were accompanied by proportional increases in supplemental sodium. Further adjustments in sodium concentration and protein content were made depending on the results of patients' serum biochemistries. To limit the osmolality of the feedings, the maximum concentration of supplemental sodium was set at 4 mEq/100 ml of formula. Supplemental bicarbonate was given if the serum bicarbonate level was <19 mEq/L and adjusted to achieve a target serum bicarbonate of 23 to 25 mEq/L.
Feedings were administered by spontaneous oral intake, supplemented by intermittent or continuous nasogastric or gastrostomy feeding tubes. Typically, infants were hospitalized at the time of initial diagnosis, and during this hospital stay, it was determined whether the patients were able to spontaneously achieve the target caloric and fluid intake. If they were not able to meet their target intake, a nasogastric tube was placed and used until the infant was able to receive a gastrostomy feeding tube. Infant formula Nepro, or Suplena with additives, was used until age 2 yr, at which time each patient was reassessed to determine the best individual diet.
Control Groups
Two control groups were used in this study: a historical population control and a literature control. The historical population controls were selected from the national Pediatric Growth Special Study of the US Renal Data Systems (USRDS) (15). The use of this national database allows for comparison of growth in the treatment group we assessed to a national standard of growth in children treated for ESRD. This special study reported on prevalent ESRD in pediatric patients followed from 1990 to 1991. The controls chosen from this large national cohort were children from all disease categories who were <1 yr of age at the time of RRT, who had received no growth hormone therapy, and who had height measurements obtained before their second birthday. The children were followed for a period of up to 1 yr from January 1990 to December 1990, and height measurements obtained 6 mo or more apart. Exclusion criteria for the control subjects included the use of growth hormone or death during the study period, or missing data regarding height measurements. More detailed information regarding the abstraction of data for this special study has been previously published (13). No information is available on the dietary management of this control group.
The literature controls were obtained from a report by Abitbol et al. (12), who described 12 infants with chronic renal failure diagnosed within the first month of life. The literature control was used to compare the effect of our nutritional approach on growth with that of a group of infants who received adequate nutrition, which is a weakness of the USRDS control group. Patients in this study were given high-caloric-density feeding regimens intended to provide ≥100% of the RDA for calories and protein. Feedings consisted of Similac PM 60/40 concentrated to 24 kcal/oz, with the addition of glucose polymers to increase the caloric density to 30 kcal/oz. Sodium supplementation was not provided. Seven of 12 patients received vitamin D therapy. All patients were followed for 2 yr. Only the final (2-yr) height change in SD scores (ΔSDS) were available for this group of patients.
Analysis of Growth
For the treatment group and the USRDS control group, height SDS was calculated by Growtrak 1.2 for Windows (Genetech, South San Francisco, CA). The height SDS was calculated by comparing the patient's height measurement to the mean US value for children of equal age and gender:
 |
The resultant SDS indicates how many SD the child is above or below the average height measurement for children of similar age and gender. A SDS of zero corresponds to the average height measurement of the general population adjusted for age and gender.
Statistical Analyses
Baseline demographic factors included race, gender, primary cause of chronic renal insufficiency (CRI), creatinine clearance (Ccr), and RRT as categorical variables. Race was classified as white, black, or other. Primary cause of CRI was divided into three broad categories: polycystic kidney disease, obstructive uropathy or dysplasia, and other causes. Obstructive uropathy or dysplasia included all causes of obstructive uropathy, renal dysplasia, and hypoplasia. Ccr in the treatment group was assessed at time of entry into study and was divided onto three groups for the purposes of analysis: <10 ml/min per 1.73 m2, 10 to 50 ml/min per 1.73 m2, and >50 ml/min per 1.73 m2. RRT was also determined at the time of entry into the study and was categorized as no RRT, dialysis, or transplant.
Because of the observational and nonrandomized study design, the comparison of the 1- and 2-yr ΔSDS between groups was performed by multivariate linear regression. Adjusted comparisons of the treatment group to the two control groups were performed to further validate the data. The treatment group and control groups were compared by the baseline characteristics, and we adjusted for variables that were considered to be significant and clinically relevant in the multivariate linear regression analysis. The comparison of the USRDS and treatment group was adjusted for RRT, primary cause of ESRD, and initial height SDS, and the outcome of the 1-yr height SDS was assessed. Because the USRDS Pediatric Growth Study was limited to 1 yr, we made no adjustment for changes in renal replacement modality, and correspondingly, in the treatment group, we made no adjustment for the institution of RRT. The comparison of the treatment group and literature control group was adjusted for the severity of disease by including Ccr as a covariate and evaluating the 2-yr height ΔSDS.
Statistical analyses were performed by SAS statistical software (version 6.12; SAS Institute Inc., Cary, NC). Dichotomous variables were analyzed by χ2 test, and continuous variables were compared by t test. In all analyses, the tests of statistical significance were two-tailed. A P value of <0.05 was considered to be statistically significant.
Results
Patient Characteristics
The treatment group consisted of 24 children treated at the University of Michigan who were diagnosed between 1992 and 1999 with polyuric renal insufficiency before 1 yr of age. All children had a Ccr of <65 ml/min per 1.73 m2 as calculated by the Schwartz equation (3). Twenty-one patients were followed for 2 yr, and 3 patients were followed for 1 yr. Data were collected regarding dialysis, transplantation, height, weight measurements, and renal function, and all data were available for all patients.
There were 12 patients in the literature control group and 42 patients in the USRDS control group. Characteristics of the groups are compared in Table 1. There were no statistical differences between the three groups with respect to race and gender. The percentage of patients with obstructive uropathy or dysplasia was similar in the treatment and literature control groups (92 versus 75%, P = NS) but was significantly less in the USRDS control group compared with the treatment group (30 versus 92%, P = 0.001).
A Ccr of <50 ml/min per 1.73 m2 was present in 92% of the treatment group, versus just 75% of the literature control group (P < 0.05). Ccr for patients in the USRDS group was not reported; however, 83% of patients in the USRDS control group were on dialysis, and 17% received transplants. RRT was instituted in 29% of the treatment group, significantly less often than in the USRDS control group (P = 0.001). During the 2-yr follow-up period, two children in the treatment group underwent preemptive transplantation, six children on dialysis received transplants, one child remained on peritoneal dialysis, one child started dialysis in the second year of life, and one child recovered renal function and dialysis was discontinued. The remaining 13 children were able to be maintained without RRT throughout the entire follow-up period.
Nutritional Status
Nutritional intakes for the study population are shown in Table 2. The average energy intake for the entire treatment group was 104 kcal/kg per day (102% RDA), and the average protein intake was 2.45g/kg per day (153% RDA). By contrast, the mean nutritional intake of the patients in the study of Abitbol et al. (12) was just 87% of the RDA for energy (despite their intended goal of providing >100% of the RDA) and 141 ± 42% of the RDA for protein intake. Dietary data were not available for the USRDS pediatric population.
Calculated Ccr for the 13 children who did not require dialysis or transplantation and only received supplemented feedings during the study period are shown in Figure 1. Initial clearances were quite impaired, <60 ml/min per 1.73 m2 in all but one child, whose clearance was 62 ml/min per 1.73 m2. After the initiation of feeds, clearance improved in most children in the first year and declined during the second year.
The weight SDS for each subject is shown in Figure 2. The baseline mean weight SDS for the entire treatment group was -0.74, -0.61 at 1-yr and -0.3 at 2-yr follow-up. The literature control group had a mean weight SDS of -0.4 at birth and -1.5 at 2 yr. The weights of the USRDS group are not shown because of the variability of weights of children on dialysis.
Linear Growth
Height measurements for all three groups are shown in Table 3 as the mean and SD for each group. There was no difference between the 1-yr height measurements of patients in the treatment and USRDS control groups. However, when the height was converted to the 1-yr height SDS, the patients in the treatment group had a greater height SDS than did the patients in the USRDS group (P = 0.001). The unadjusted 1-yr height ΔSDS showed no difference between the treatment and USRDS control groups. The unadjusted 2-yr height ΔSDS of the literature control group was significantly lower than the treatment group (P = 0.002).
The initial multivariate analysis compared the treatment group with the USRDS control group at 1 yr (Table 4). This model simultaneously assessed the effect of treatment on growth while controlling for the severity of disease by RRT, primary cause of ESRD, and initial height SDS; the model accounted for 44% of the variance in the 1-yr ΔSDS by linear regression analysis. There was evidence of a treatment difference between the treatment and USRDS control groups in predicting the 1-yr height SDS while adjusting for the severity of disease, primary cause of ESRD, and initial height SDS (P < 0.012). The predicted 1-yr height ΔSDS was greater for the patients in the treatment group than the patients in the USRDS control group, with an increase in height ΔSDS of +1.37, assuming the RRT and diagnosis were the same (Table 4).
Children on dialysis who were not receiving RRT had significantly lower 1-yr height ΔSDS than did members of the transplant group, with the no-RRT group having the lowest predicted 1-yr height ΔSDS compared with children who had received a transplant. Children with polycystic kidney disease and other causes of renal insufficiency had little difference in predicted 1-yr height ΔSDS compared with the referent group of children with obstructive uropathy. When only children with obstructive uropathy from the USRDS control group were used for analysis, we found a statistically significant difference in 1-yr height ΔSDS in the treatment group compared with the USRDS control group, with an increase in height ΔSDS of +1.74 in the treatment group compared with the control group, assuming similar RRT (Table 4). This model accounted for 47% of the variance in the 1-yr ΔSDS by linear regression analysis.
Additional analyses compared the treatment group with the literature control group at 2 yr. A linear regression model to assess the outcome of the 2-yr height ΔSDS (2-yr height SDS minus initial height SDS), while adjusting for the Ccr, revealed a statistically significant greater 2-yr height ΔSDS in the treatment group than the control group (P < 0.03). This model accounted for 34% of the variation of the 2-yr height ΔSDS. The literature control group had a decrease in the predicted 2-yr height ΔSDS by 1.83 compared with the treatment group (Table 3). In children with the lowest calculated Ccr, there was a small gain in 2-yr height ΔSDS among both treatment and control groups compared with those children with clearances >50 ml/min per 1.73 m2, which was not statistically significant. The addition of primary cause of ESRD as a covariate had no additional effect on the model, with the treatment group continuing to have significantly greater height ΔSDS (data not shown).
Discussion
In this study, we describe the beneficial effect of a dilute, sodium-supplemented, high-volume feeding regimen on linear growth in young children with severe polyuric CRI. Patients treated with this specified nutritional regimen maintained their height SDS at 1 and 2 yr despite significant renal insufficiency. The treatment group was able to maintain a nearly normal height SDS, with the height SDS not decreasing over time, as is typical for children with ESRD. Instead, there was a net gain of height SDS of 0.15 during the 2 yr. These results were superior to those seen in two previously reported comparable groups of children, one group with CRI treated with highcaloric-density feedings, and the other with ESRD and RRT. This gain of height SDS is unusual in infants with CRI, who typically demonstrate a loss of height SDS over time (16).
The natural history of poor growth commonly seen in children with CRI is clearly illustrated by the initial and 2-yr height SDS of patients in the literature control group, who had poor growth despite supplementation with high-density feeds (12). The nutritional intake of the treatment group consisted of a higher volume of sodium-supplemented feeds with similar energy and protein intakes than the literature control group. Compared with this group, the patients we studied demonstrated stable growth during 2 yr, even though the mean Ccr was significantly lower than in the group reported by Abitbol et al. (12). The weight SDS of the treatment group was also higher at 2 yr than the literature control group. Some patients had been on dialysis or had received transplants, which may have affected the weight gain. The trend of the weight SDS, however, continued to improve during the 2 yr, so it is unlikely that this solely represents fluid gain.
The USRDS population control group was used to compare our results with the prevailing standard of care of young children with ESRD. The initial height SDS was lower in the USRDS control group than the treatment group. This difference in initial height SDS can be explained partly by the severity of renal disease, and also by primary cause of ESRD; however, we adjusted for these factors in the analysis. Height SDS of the USRDS group was maintained during the 1-yr follow-up period and represented the average height SDS among prevalent pediatric dialysis and transplant patients. The maintenance of the mean height SDS is partially explained by the contribution of a gain in height by transplant patients of 0.13 height SDS per year for this age group (13). However, the USRDS control group reflects an ESRD population treated with RRT that was able to maintain height SDS but demonstrated no catch-up growth, highlighting the need for a method to improve growth in children with chronic renal failure before they require transplantation.
This retrospective study of nutritional intervention adjusts for the clinically significant differences of severity of disease and the primary cause of CRI in the analyses. Demographic variables (race and gender) did not vary by group: the causes of congenital ESRD in this age group are similar throughout the United States (15). The internal validity of the study is further strengthened by the use of two control groups with follow-up for a 2-yr period. However, the center effect and the potential differences in the care of children in the USRDS database could not be taken into consideration in this analysis. We were also limited by our inability to retrospectively collect data on growth factors such as insulin-like growth factor, which might have given clues to possible biologic mechanisms of growth failure in this subset of young children with ESRD. Linear growth was routinely measured; however, skinfold thickness and midarm circumference were not routinely measured, so changes in muscle mass could not be assessed.
Sodium has been reported to be vital for growth in children, specifically very low birth weight infants, children with Bartter's syndrome, and children with salt-wasting gastroenteropathy (17,18,19). Sodium depletion is known to affect not only extracellular volume, but also growth and nitrogen retention (20). Sodium intake also promotes the normal expansion of extracellular fluid volume that is necessary for muscle development and mineralization of bone (18). Fine et al. (21) have shown in a rat model that the sodium requirements for growth are unrelated to body size because the sodium is primarily used for new tissue growth. They noted a decrease in extracellular fluid volume with severe salt restriction. The authors concluded that plasma volume expansion appears to be required for growth, and salt deprivation may in part affect growth by depleting extracellular fluid volume. We hypothesize that this same process occurs in children with polyuric, salt-wasting chronic renal failure and that the repletion of sodium and water losses is vital to promote normal growth in these children.
To our knowledge, this is the largest reported series of children with chronic renal failure rigorously treated with sodium and water supplementation and the most comprehensive comparison available of the potential benefits of sodium and water supplementation on growth. Our method of nutritional intervention with a dilute, high-volume sodium supplemented feeding regimen was more successful in improving growth than were previously reported series of nutritional intervention in children with severe CRI (11,12). The stabilization of growth in the treatment compared with the control groups of patients demonstrates the ability of sodium and water supplementation to overcome typical growth retardation seen in infants and children with chronic renal failure. The specified nutritional prescription provided to these patients is a simple recipe that can be easily generalized to all polyuric congenital causes of CRI.
The importance of nutritional supplementation in children with chronic renal failure was recently highlighted in publications from the group at Great Ormond Street (22,23), which demonstrated catch-up growth in children with chronic renal failure after provision of supplemental enteral nutrition. Their results are difficult to compare with ours because enteral nutrition in their study was not initiated until growth failure had already occurred, and because they did not report detailed information regarding the composition, especially caloric density and sodium supplementation, which was commonly provided (S. Ledermann, personal communication), of the feedings their patients received. Their results, however, do demonstrate that meticulous nutritional support is a key component of the successful management of the child with chronic renal failure, and such support can often improve growth in growth-retarded children without the addition of growth hormone.
The estimated Ccr seen after the initiation of feeds was improved in the 13 patients who received sodium supplemented feedings only. In addition, four patients experienced worsening of renal function in their second year or were preemptively transplanted. Analysis of these data suggests that the use of sodium-supplemented feedings may be also a therapeutic intervention to transiently improve the GFR, allowing deferment of RRT and facilitating planning for a preemptive renal transplant.
The overall cost of sodium and water supplementation added to the usual cost of dietary supplements is only cents per day, or an estimated $50 per year. The use of table salt and baking soda makes it possible for families to inexpensively prepare these supplements at home (Appendix). The current average wholesale cost of growth hormone is $420 for a 5-mg/ml vial (24), and the annual cost of growth hormone can be as high as $15,000. Clearly, sodium and water supplementation is inexpensive compared with growth hormone therapy or dialysis.
We conclude that specified nutritional therapy consisting of sodium and water supplementation can maintain or improve the growth of children with polyuric causes of CRI. This approach not only promotes improved growth rates in this patient population, but also likely delays the need for dialysis until preemptive renal transplantation can be accomplished. This cost-effective form of nutritional intervention to improve growth can be administered in any clinical setting and may be particularly suited to regions of the world where dialysis is either technologically or financially impossible.
Appendix: Instructions for Sodium Chloride and Sodium Bicarbonate Solutions
Salt solutions can be made inexpensively at home and then can be measured out in small amounts to be added to formulas. However, you must be extremely careful with these solutions: if given to a child undiluted, the concentration of the solution could make them very sick. Please make the recipes carefully—mark the bottles carefully and only have one person responsible for making them. Never give these solutions to a child without diluting them in some other fluid.
NaCl Solution (Salt Solution)
Take 1/3 cup table salt (without iodine) and mix with 1 quart water. Store in refrigerator. Discard after 2 wk and make a new batch.
1 ml = 1 mEq
The prescription for your child is to add _____ ml of NaCl solution to _____ ml of _____ times daily.
NaHCO3 Solution (Sodium Bicarbonate Solution)
Add one 8-ounce box of Arm and Hammer baking soda to 3 quarts of water. Store in refrigerator. Keep tightly capped. Discard after 2 wk and make a new batch.
1 ml = 1 mEq
If you can find a 4-ounce box of baking soda or you have a scale, you can measure out 4 ounces and add to 11/2 quarts of water to save room.
The recipe for your child is to add _____ ml NaHCO3 solution to each _____ ml of _____ (must be in water or a nonacid drink) _____ times a day.
Acknowledgments
This work was supported by the American Kidney Fund/Amgen Clinical Scientist in Nephrology Fellowship Award. We thank Dr. R.A. Wolfe for his advice on the statistical analyses and Dr. F.K. Port for his review of the article in manuscript. Some of the data reported here were supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government. Presented in abstract form at the ASN meeting in New Orleans, Louisiana, October 1996.
References
- Abitbol C, Chan JC, Trachtman H, Strauss J, Greifer I: Growth in children with moderate renal insufficiency: Measurement, evaluation, and treatment [Review]. J Pediatr129 : S3-S8,1996
- Karlberg JSF, Hennicke M, Wingen A, Rigden S, Mehls O, European Study Group: Nutritional treatment of chronic renal failure in childhood. Pediatr Nephrol 10:283 -287, 1996[Medline]
- Hellerstein SHM, Grupe WE, Fine RN, Fennell RS, Chesney RW, and Chan JCM: Nutritional management of children with chronic renal failure. Summary of the task force on nutritional management of children with chronic renal failure. J Pediatr 1:195 -211, 1987
- Sedman AS, Friedman A, Boineau F, Strife CF, Fine R: Nutritional management of the child with mild to moderate chronic renal failure [Review]. J Pediatr 129:S13 -S38, 1996
- Rigden SP, Start KM, Rees L: Nutritional management of infants and toddlers with chronic renal failure. Nutr Health5 : 163-174,1987[Medline]
- Holliday MA, ET, Morris CR, Jarrah AS, Harrah JL: Pitressin-resistant hyposthenuria in chronic renal disease. Am J Med 42: 378-387,1967[Medline]
- Tejani ACL, Sullivan EK: A longitudinal study of the natural history of growth post-transplantation. Kidney Int49 [Suppl]: 103-108,1996[Medline]
- Kaiser BA, Polinsky MS, Stover J, Morgenstern BZ, Baluarte JH: Growth of children following the initiation of dialysis: A comparison of three dialysis modalities. Pediatr Nephrol8 : 733-738,1994[Medline]
- Hokken-Koelga ACS, Van Zaal AE, De Ridder MA, Wolff ED, De Jong MCJW, Donckerwolke RA, De Muinck Keizer-Schrama SMPF, Drop SLS: Growth after renal transplantation in prepubertal children: Impact of various treatment modalities. Pediatr Res 35:367 -371, 1994[Medline]
- Wolfe RA, Port FK, Webb RL, Bloembergen WE, Hirth R, Young EW, Ojo AO, Stawderman RL, Gillespie B, Held PJ, Parekh R, Stack A, Tedeschi PJ, Loos E, Hulbert-Shearon T, Ashby VB, Callaard S, Orzol SM, Carrol CC, Wheeler JR, Jones CA, Frederick PR, Greer JW, Agodoa LYC: Excerpts from the United States Renal Data System 1998 Annual Data Report. Am J Kidney Dis 32[Suppl 1]:S1 -S162, 1998[Medline]
- Brewer E: Growth of small children managed with chronic peritoneal dialysis and nasogastric tube feedings: 203-month experience in 14 patients. Adv Perit Dial 6:269 -272, 1990[Medline]
- Abitbol CL, Zilleruelo G, Montane B, Strauss J: Growth of uremic infants on forced feeding regimens. Pediatr Nephrol7 : 173-177,1993[Medline]
- Turenne MN, Port FK, Strawderman RL, Ettenger RB, Alexander SR, Lewy JE, Jones CA, Agodoa LYC, Held PJ: Growth rates in pediatric dialysis patients and renal transplant recipients. Am J Kidney Dis 30: 193-203,1997[Medline]
- National Research Council. Subcommittee on the Tenth Edition of the RDAs. Recommended Dietary Allowances, 10th Ed., Washington, DC, National Academy Press, 1989
- National Institutes for Health. US Renal Data System 1998 Annual Data Report, Bethesda, National Institute of Diabetes and Digestive and Kidney Disease, 1998
- Rizzoni G, Basso T, Setari M: Growth in children with chronic renal failure on conservative treatment. Kidney Int26 : 52-58,1984[Medline]
- Wassner S, Kulin HE: Diminished linear growth associated with chronic salt depletion. Clin Pediatr (Phila)29 : 719-721,1990
- Ray PE, Lyon RC, Ruley EJ, Holliday MA: Sodium or chloride deficiency lowers muscle intracellular pH in growing rats. Pediatr Nephrol 10:33 -37, 1996[Medline]
- Fine BP, Ty A, Lestrange N, Maher E, Levine OR: Diuretic-induced growth failure in rats and its reversal by sodium repletion. J Pharmacol Exp Ther 242:85 -89, 1987[Abstract/Free Full Text]
- Wassner S: Altered growth and protein turnover in rats fed sodium-deficient diets. Pediatr Res26 : 608-613,1989[Medline]
- Fine BP, Ty A, Lestrange N, Levine R: Sodium deprivation growth failure in the rat: Alterations in tissue composition and fluid spaces. J Nutr 117:1623 -1628, 1987
- Ledermann SE, Shaw V, Trompeter RS: Long-term enteral nutrition in infants and young children with chronic renal failure. Pediatr Nephrol 13:870 -875, 1999[Medline]
- Kari JA, Gonzales C, Ledermann SE, Shaw V, Rees L: Outcome and growth of infants with severe chronic renal failure. Kidney Int 57:1681 -1687, 2000[Medline]
- 2000 Drug Topics Red Book, 465th Ed., Oxford, Blackwell Science, 2000. Received for publication June 1, 1999. Accepted for publication May 25, 2001.
|
Ћечение содой-6 |
ƒневник |
»сточник http://www.plasma.com.ua/chemistry/chemistry/soda.html
ћедицина.

ак выгл€дит сода, прекрасно знают все - это белый порошок, который впитывает воду и отлично в ней раствор€етс€. Ќо мало кто знает об удивительных целебных свойствах этого «простого» вещества. ћежду тем, сода - гидрокарбонат натри€ - один из главных ингредиентов нашей крови. –езультаты исследовани€ вли€ни€ соды на организм человека превзошли все ожидани€. ќказалось, что сода способна выравнивать кислотно-щелочное равновесие в организме, восстанавливать обмен веществ в клетках, улучшать усвоение кислорода ткан€ми, а также преп€тствовать потере жизненно необходимого кали€. ѕомогает сода при изжоге, при морской болезни, при простудах, при сердечных заболевани€х и головных бол€х, при кожных заболевани€х. ак видите, сода - лекарство первой помощи.
–аствор питьевой соды используетс€ в качестве слабого антисептика дл€ полосканий, а также как традиционное кислотонейтрализующее средство от изжоги и болей в желудке (современна€ медицина не рекомендует примен€ть из-за побочных эффектов, в том числе, из-за «кислотного рикошета») или дл€ устранени€ ацидоза и т. п.
ѕищева€ сода примен€етс€ дл€ лечени€ заболеваний, св€занных с повышенной кислотностью; раствор питьевой соды примен€етс€ дл€ полоскани€ горла, дл€ промывани€ кожи при попадании кислот.
Ѕикарбонат натри€ (пищева€ сода) может замедл€ть развитие хронического заболевани€ почек. такому выводу пришли ученые из оролевской клиники Ћондона (Royal London Hospital), ¬еликобритани€. ќни исследовали 134 человека с запущенным хроническим заболеванием почек и метаболическим ацидозом.
ќдна группа испытуемых проходила обычное лечение, а втора€ помимо традиционного лечени€ ежедневно получала небольшое количество пищевой соды в виде таблеток. ” тех больных, кто пил бикарбонат натри€, функции почек ухудшались на 2/3 медленнее, чем у прочих.
Ѕыстрое прогрессирование заболевани€ почек наблюдалось только у 9% подопытных из «содовой группы» против 45% испытуемых, лечившихс€ традиционно. роме того, у принимавших соду реже развивалась терминальна€ стади€ почечной недостаточности, котора€ требует диализа. ѕримечательно, что повышение содержани€ бикарбоната натри€ в организме не вызывало у больных повышени€ кров€ного давлени€.
Cода €вл€етс€ недорогим и эффективным средством лечени€ хронического заболевани€ почек. ќднако исследователи предостерегают: прием соды должен проходить под наблюдением врача, который должен правильно рассчитать дозировку дл€ больного.
Ћечебные свойства пищевой соды.
–аньше гидрокарбонат натри€ примен€лс€ очень широко (как и другие щелочи) в качестве антацидного средства при повышенной кислотности желудочного сока, €звенной болезни желудка и 12-типерстной кишки. ѕри приеме внутрь пищева€ сода быстро нейтрализует сол€ную кислоту желудочного сока и оказывает выраженный антацидный эффект. ќднако применение соды заключаетс€ не только в блест€ще отмытой посуде и избавлении от изжоги. ѕищева€ сода занимает достойное место в домашней аптечке.
ак и древние египт€не, получавшие природную соду из озерных вод методом выпаривани€, люди использовали и другие свойства соды. ќна обладает нейтрализующими качествами, используетс€ в медицинской практике дл€ лечени€ гастритов с повышенной кислотностью. —пособна убивать микробов, используетс€ как дезинфицирующее средство: соду примен€ют дл€ ингал€ций, полосканий, очищени€ кожи.
Ўирокое применение сода имеет и в здравохранении.
ѕрофилактика кариеса.
ислоты, образующиес€ во рту в результате жизнеде€тельности бактерий, разрушают эмаль зубов. Ёти кислоты можно нейтрализовать, несколько раз в день полоща рот раствором пищевой соды. ћожно поступить иначе: смочите зубную щетку водой, опустите ее в соду и почистите зубы. —ода, кроме того, оказывает легкое абразивное действие: она отполирует зубы, не поврежда€ эмали.
ќт непри€тного запаха ног.
ƒобавленна€ в воду дл€ ножной ванны сода нейтрализует выдел€емые бактери€ми кислоты, которые и придают ногам непри€тный запах. —ода поможет также устранить резкий запах пота под мышками.
ѕри укусах насекомых.
Ќе расчесывайте до крови укусы комаров и прочих кровососов. Ћучше приготовьте кашеобразную смесь из воды и соды и нанесите на место укуса. —одова€ кашица облегчит также зуд, вызванный ветр€ной оспой или контактом кожи с борщевиком, крапивой.
ѕри опрелост€х.
—одовые примочки значительно улучшают состо€ние малышей с опрелост€ми. ќни уменьшают зуд и ускор€ют заживление кожи.
ѕри цистите.
Ѕолезнетворные бактерии живут в мочевом пузыре в слегка кислой среде. ≈сли ваш мочевой пузырь пал жертвой инфекции, идеальный послеобеденный напиток дл€ вас - шипучий коктейль из пищевой соды с водой.
ѕри солнечных ожогах.
ƒобавьте в теплую ванну немного пищевой соды: она см€гчит воду, превратив ее в успокаивающую примочку дл€ раздраженной кожи.
ќт боли в горле.
–азмешайте 0,5 чайн. ложки соды в стакане воды и каждые 4 часа полощите горло приготовленным раствором: он нейтрализует кислоты, вызывающие боль. ѕолоскание таким раствором рта поможет сн€ть и воспаление слизистой ротовой полости.
ќт непри€тного запаха изо рта.
¬ сочетании с перекисью водорода пищева€ сода дает мощный окислительный эффект и разрушает бактерии, порождающие непри€тный запах во рту. ƒобавьте 1 стол. ложку соды в стакан раствора перекиси водорода (2-3%) и прополощите рот.
ѕри простуде.
ѕолезно делать ингал€цию. ƒл€ этого можно вз€ть небольшой чайник, вскип€тить в нем 1 стакан воды с 1 чайн. ложкой соды. —делать из твердой бумаги трубочку, надеть ее на носик чайника и вдыхать пар в течение 10-15 минут. ƒанна€ ингал€ци€ очень помогает дл€ отделени€ мокроты.
ƒл€ отхаркивани€ в€зкой мокроты 2 раза в день выпивать натощак по 1/2 стакана теплой воды, в которой растворены 0,5 чайн. ложки соды и щепотка соли.
ѕри частых мигрен€х.
аждый день принимать раствор кип€ченой воды с пищевой содой. ¬ 1-й день за 30 минут до обеда выпивать 1 стакан раствора (0,5 чайн.ложки соды + вода), 2-й день - 2 стакана и т.д., довед€ до 7 стаканов. ѕосле уменьшать дозу в обратном пор€дке.
ѕрочее.
 ѕри ринитах, стоматитах, ларингитах, конъюнктивитах примен€ют 0,5-2% раствор соды.
ѕри ринитах, стоматитах, ларингитах, конъюнктивитах примен€ют 0,5-2% раствор соды.
ƒл€ обеззараживани€ слизистой оболочки рта полезно полоскать рот некрепким раствором (сода - 85 г, соль - 85 г, мочевина - 2,5 г) после еды.
—редство от курени€: полоскать рот раствором пищевой соды (1 столова€ ложка на 200 мл воды).
ѕри сухости кожи, сухих дерматитах, ихтиозе и псориазе полезны лечебные ванны (сода - 35 г, карбонат магнезии - 20 г, перборат магни€ - 15 г). “емпература воды должна быть не выше 38-39° —, сначала нужно садитьс€ просто в теплую ванну, потом постепенно увеличивать температуру. ƒлительность ванны 15 минут.
|
—ода - лекарство от многих болезней. —ода пищева€, лекарство, болезнь, лечение, вода, человек, здоровье |

–оль соды и щелочей в защите здоровь€ людей и растений ѕравильно, что не забываете про соду. Ќе без причины ее называли пеплом божественного ќгн€. ќна принадлежит к тем, широко даваемым л
|
—ода и рак |
Ёто цитата сообщени€ KonVeda [ѕрочитать целиком + ¬ свой цитатник или сообщество!]
–ак и ... обычна€ сода.
ѕредлагаем вашему вниманию перевод статьи ƒэвида јйка, оригинал которой на английском вы можете найти на сайте davidicke.com
÷ифры, конечно, впечатл€ющие. ¬осемь миллионов людей умирает ежегодно от рака во всем мире, только в —Ўј – это более пол миллиона. ќжидаемый рост смертности к 2030 году – 12 миллионов. –ак €вл€етс€ самой распространенной причиной смерти в возрастной группе до 85 лет. ¬ Ўтатах от этой болезни умирает каждый четвертый человек. аждый четвертый! ћы лишились многих своих свобод, когда согласились, чтобы нас «защищали от терроризма», люди продолжают болеть и умирать от недугов, которые элитные семьи и их фармацевтические картели отказываютс€ лечить.

|
Ѕез заголовка |
Ёто цитата сообщени€ KonVeda [ѕрочитать целиком + ¬ свой цитатник или сообщество!]

’очу вам предложить домашнюю маску дл€ волос, которую вы без труда сможете самосто€тельно приготовить. ћаска не только питает волосы, но и лечит их и что самое главное, значительно ускор€ет рост волос. ћаска испытана мною на себе и поэтому € с уверенностью могу сказать, что результат просто великолепный, волосы растут в 3 – 4 раза быстрее, они станов€тс€ послушными, улучшаетс€ их состо€ние, по€вл€етс€ здоровый блеск, волосы выпадают все меньше и меньше.
√лавное в этом деле набратьс€ терпени€, поскольку курс лечени€ этой маской составл€ет 1,5 мес€ца.
|
Ѕез заголовка |
Ёто цитата сообщени€ KonVeda [ѕрочитать целиком + ¬ свой цитатник или сообщество!]

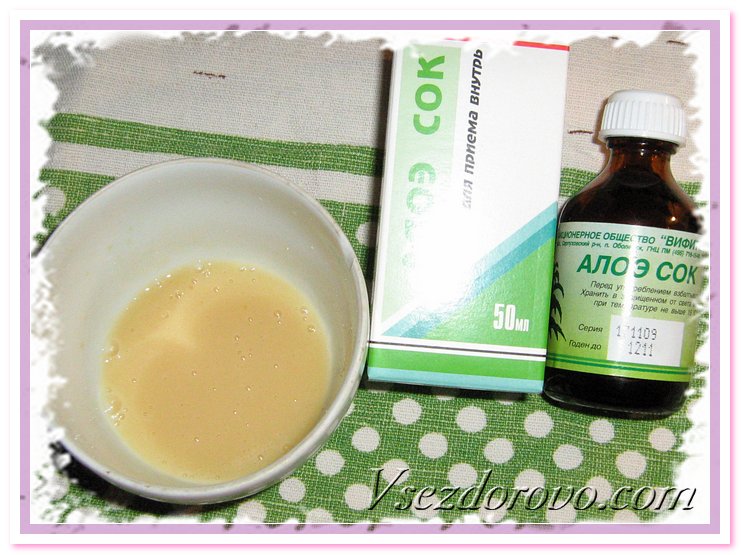
2 ст.л. сока свежего картофел€
2 ст.л. сока алоэ ( биостимулированный)
1 желток
Ќаносим на волосы и на час пр€чем по теплую шалочку, затем смываем. ќчень актуальна маска именно сейчас когда закончилс€ сезон натуральных витаминов и волос€ные потери очень ощутимы)) ≈ще советую кончики смазать оливковым маслом, если они у вас сухие.
|
Ѕез заголовка |
Ёто цитата сообщени€ KonVeda [ѕрочитать целиком + ¬ свой цитатник или сообщество!]

|
ћј“≈–»јЋџ
|
—ѕќ—ќЅ »«√ќ“ќ¬Ћ≈Ќ»я
|
–ецепт вышеописанного крема сделан по подсказке дочери, в свое врем€ закончившей курс косметологов-визажистов в школе Burda Moden. рем имеет при€тную консистенцию, легкий аромат жасмина, обладает см€гчающим действием.
’ранитс€ в закрытой таре при комнатной температуре. ¬ холодильник ставить не об€зательно, в качестве консерванта -лимонный сок. ћожно использовать как крем-маску на ночь, надева€ перчатки.
≈сли кому-то интересно, вот информаци€ о составл€ющих крема:
ћасло манго -получают из косточек плодов дерева манго. ≈го отличает от масел других сем€н наличие в его составе большого количества неомыл€емых жиров. Ќеомыл€ема€ фракци€ масла манго содержит важные питательные вещества, витамины и фитонутриенты, способствующие быстрому заживлению кожи. »спользование этого натурального средства может помочь справитьс€ со многими проблемами кожи, включа€ пигментные п€тна, морщины, раздражени€, солнечные ожоги, небольшие повреждени€ кожи, экзему, аллергические реакции, укусы насекомых, легкие обморожени€ и т.д.
¬итамины ј и ≈ (аевит)- отвечают за защиту кожи, ее обновление и хорошую структуру.
√лицерин -способен удерживать некоторое количество воды, предохран€€ кожу от чрезмерного высушивани€. ƒобавл€етс€ в крем дл€ большей однородности.
ћасло жасмина оказывает благотворное действие на сухую и чувствительную кожу, повыша€ ее эластичность
|
Ѕез заголовка |
Ёто цитата сообщени€ Jelly77 [ѕрочитать целиком + ¬ свой цитатник или сообщество!]
¬олшебный крем дл€ лицаЁ“ќ“ ѕќ—“ я ѕ–ќ÷»“»–ќ¬јЋј ƒј¬Ќќ ” ћј–“»Ќџ 25. ƒ≈¬ќ„ »!!! ћќ∆≈“ ќћ”-“ќ ѕ–»√ќƒ»“—я ¬ ’ќ«я…—“¬≈!!!! –≈ћ ќЅјЋƒ≈ЌЌџ…!!! ѕќЋ№«”ё—№!! |
|
"—начала предисловие. Ћет так …дцать назад,совсем давно,када € была еще юна€ совсем (ЁЁЁ’… ),€ читала один женский роман. “ам повествовалось о даме 42 лет,котора€ считала себ€ еще молодой девушкой и выгл€дела соответственно!!! » там же давалс€ подробный рецепт крема,которым она пользовалась,когда коже была нужна экстренна€ поддержка.я еще, помню, очень удивилась:надо же,рецепт крема в художественной книге. Ќо так как € всеже посчитала бредом,что в 42 можно молодо выгл€деть…(€ думала, что вообще не доживу до сей др€хлости ),то и рецепт списывать € не стала. Ќо!!! аким-то волшебным образом название этой книги осталось у мен€ в голове.
ѕрошло хм…ну ооочень много лет. Ѕуквально мес€ц назад € поймала себ€ на мысли,что отражение в зеркале уже не радует. —тали закрадыватьс€ различные мыслишки,типа:прощай,молодость…природу не обманешь…ну и проча€ очень грустна€ дребедень. я даже подумала впасть по этому поводу в депрессию,но, слава Ѕогу, передумала. » тогда и всплыло у мен€ откуда-то из закоулков пам€ти название этого женского романа и то,что там есть чудодейственный рецепт.

—овпадение или нет,что сейчас € как раз ровесница его героини? Ќаверное, не совпадение. я бросилась искать эту книгу в интернете,нашла,и выудила оттуда вожделенный рецепт,который и предъ€вл€ю сейчас вашему вниманию:
÷итата из книги —юзан убелки «ћадам придет сегодн€ позже»
«ѕотом изготовл€ю свой знаменитый крем против
морщин, который за неделю разгладит самую плохую кожу. Ќе зр€ же у мен€ в сорок два года гладкое лицо. —амое главное – не есть м€са, но уход тоже важен! ѕоэтому свои кремы € делаю сама. ’от€ дл€ создани€ идеального рецепта мне понадобилось два года, и здесь срабатывает истина, что все великое совсем, совсем просто.
—екрет моей гладкой красивой кожи предельно прост: майонез с настоем из ромашки!
–омашка разглаживает кожу как гор€чим утюгом, а майонез питает и придает ей упругость. ≈сли кожа испорчена, как у мен€, солнцем и ветром или больше не реагирует на покупные кремы, бегом к плите и как следует размешивать. ћайонез с ромашкой справитс€ с ней.
¬ п€ть часов пополудни, после стакана морковного сока, € начинаю св€щеннодействовать.
ƒелаю майонез из миндального масла и половинки желтка, это очень просто. ƒобавл€ю туда настой из ромашки, столько, чтобы не загустело. “уда же немного морской соли, каплю меда.
–астапливаю на вод€ной бане две кофейные ложечки вазелина. огда он становитс€ жидким и чуть остывает, добавл€ю к нему майонез и размешиваю ручным миксером. ≈ще теплым сливаю все в маленький горшочек и ставлю в морозилку. „ерез п€ть минут все манипул€ции закончены!
¬ечером ополаскиваю лицо настоем из ромашки, беру немного крема из горшочка и втираю во влажную кожу. ѕотом вбиваю его пальцами досуха. ќсвежает великолепно! огда бы кожу ни ст€нуло, € повтор€ю процедуру. „ерез четыре дн€ шелушение исчезает, мелкие
морщинки тоже, большие поры закрываютс€, € выгл€жу розовой и свежей. “еперь второй трюк.
Ѕольшим и указательным пальцами массирую свои брови, все врем€ в одном направлении – от переносицы к вискам, семь-восемь раз, пока не покраснеет кожа. ѕотом, с закрытыми глазами, расчесываю брови щеткой. Ёто расслабл€ет маленькие мышцы, образующие морщины, снимает напр€жение со лба и разглаживает его.
“еперь это должно стать об€зательной ежевечерней процедурой перед сном. » каждый раз, когда сморщу лоб. ¬едь морщины делаешь себе сам неправильной мимикой.»
я срочно изготовила этот крем. “олько масло вз€ла не миндальное,а оливковое,но самое хорошее-экстра вижн. ћайонез как таковой у мен€ не получилс€,получилась масл€ниста€ бела€ масса,с коей € и проделывала последующие манипул€ции. » вазелина €, наверное, мало добавила,потому что крем на выходе получилс€ очень жидким и не застыл совсем. ј застыть он должен, € думаю, за счет вазелина. ¬азелин € брала косметический.
ѕримен€ла крем точно по рецепту,то есть после опаласкивани€ лица ромашкой. ѕравда, использовала € его не только на ночь,но и как дневной. Ќо € ведь дома сижу,а кому на работу - не советую это делать,так как под маки€ж он не годитс€. ¬питываетс€ долго,даже больше часа,потому нужно им пользоватьс€ часа за два до сна. ѕервый результат € увидела буквально дн€ через три!!! Ћицо заметно посвежело и подт€нулось. —ейчас € перешла пока на другой ночной крем,потому что этот на оливковом масле всеже т€желоват немного дл€ моей кожи (но это € уже вредничаю,действует он прекрасно! ). —ледующую порцию сделаю, как и положено, на миндальном масле,оно должно в аптеках продаватьс€. “олько не знаю, сколько его надо,чобы майонез на нем сделать . ¬ общем,девочки,рецепт несложный и поистине волшебный".
автор текста:≈лена ’озина
рем:
»нгредиенты:
- половина свежего желтка
- 2 ч.л миндального масла (€ думаю можно заменить оливковым)
- 1 ч.л. морской пищевой соли
- 2 ч.л. отвара ромашки
- 0.5 ч.л. меда
- 2 ч.л. вазелина
—пособ изготовлени€:
ѕоскольку в книге не указаны пропорции, € сделала их, опира€сь на свой опыт. » несмотр€ на то, что мне всего 30 лет, € его испробовала на себе. ожа после этого крема очень хороша€. —мешала все ингридиенты сразу, не растаплива€ вазелин и блендером не перемешивала, иначе это количество крема размазалось бы по стенке. ¬азелин не растворилс€ ,поэтому € поставила в морозилку, и когда он немного прихватилс€, € тщательно все смешала ложечкой до однородной массы. » так несколько раз. “еперь он там и стоит. Ѕеру по утрам немного и делаю все так, как сказано выше, только через минут 30 смываю остатки ватным тампоном, смоченным в минералке. “ому, кто с уважением относитс€ к своей коже, крем должен понравитс€. ќчень рекомендую))
автор поста:софико80
http://www.by-hand.ru/item/view/9291
|
Ўампунь самодельный |
ƒневник |

–ецепт этого шампун€ € нашла среди народных средств дл€ волос и только немного дополнила его, прибавив некоторые ингредиенты. Ќесмотр€ на то, что в него не вход€т мыл€щие вещества, шампунь хорошо промывает волосы. ј главное – укрепл€ет их, питает, уменьшает образование перхоти. ѕриготовить его несложно. ƒл€ того, чтобы не тратить на этот шампунь врем€ вс€кий раз, когда он понадобитс€, € делаю сразу несколько порций.
»нгредиенты на три порции шампун€ (дл€ волос средней длины):
одна пачка желатина (20 г);
два желтка;
сушеные крапива, череда и календула – по чайной ложке с горкой;
кружка кип€тка;
половина чайной ложки репейного масла;
эфирное масло – по желанию.
¬скип€тите воду и заварите в кружке крапиву, череду и календулу. Ёти травы уменьшают образование перхоти, питают кожу. Ќакройте кружку блюдцем и дайте настою остыть. √отовый настой процедите.

¬ небольшую кастрюльку высыпьте желатин, залейте холодным трав€ным настоем. ќставьте на полчаса, чтобы желатин набух. ѕодогрейте на медленном огне, помешива€ и не довод€ до кипени€. —нова остудите.

ƒва желтка тщательно отделите от белков. Ѕелка в шампуне быть не должно – при контакте с гор€чей водой он заваритс€ и останетс€ в волосах непри€тными белыми сгустками.

ƒобавьте к желткам репейное масло. ≈сли у вас суха€ кожа головы, кладите половину чайной ложки или даже чуть больше. ≈сли жирна€, от масла можно отказатьс€ вообще. ћожно добавить эфирное масло дл€ запаха. ќднако учитывайте, что эфирные масла сушат кожу, поэтому класть его нужно буквально каплю, не больше.
¬збейте желтки с маслом. ќстывший и процеженный желатин влейте в желтки – сначала по одной ложке, взбива€, пока не получите однородную массу, потом все остальное.

Ўампунь готов к употреблению. ƒве порции перелейте в силиконовые формы или в другую удобную емкость и поставьте в морозильник, а третью можно пускать в дело. «амороженный шампунь может хранитьс€ в морозильнике до шести мес€цев. ѕеред употреблением его нужно разморозить – до состо€ни€ желе при комнатной температуре либо до жидкого состо€ни€ на вод€ной бане.
Ўампунь нанесите на мокрые волосы, вотрите в кожу головы и оставьте на дес€ть минут. «атем смойте его прохладной водой.

|
ћаска от морщин |
ƒневник |
[„удо] действенна€ маска-крем от морщин
»сточник http://arkalika.ru/2011/02/chudodejstvennaya-maska...-morshhin/#.Tw1QZgTKRew.custom
 ћногие производители дорогих кремов и масок обещают избавление от морщин за короткие сроки. Ќевозможно проверить все средства, да и многим это будет не по карману. ќднако можно сделать чудодейственную крем-маску от морщин своими руками.
ћногие производители дорогих кремов и масок обещают избавление от морщин за короткие сроки. Ќевозможно проверить все средства, да и многим это будет не по карману. ќднако можно сделать чудодейственную крем-маску от морщин своими руками.
—егодн€ мы рассмотрим более подробную технику изготовлени€ домашних масок на основе базовых и эфирных масел. —вой выбор € решила остановитьс€ на крем-маске от морщин дл€ всех типов кожи. омпоненты выбраны благодар€ их уникальным свойствам и возможности их достать.
ќпишу компоненты маски-крема и дл€ нагл€дности и убеждени€ свойства, которыми они обладают.
Ѕазовые масла:
1) 5мл. абрикосового масла (1 ч.л.)
- ќказывает противовоспалительное, регенерирующее, тонизирующее, омолаживающее кожу действие, а также питающее, см€гчающее, увлажн€ющее действие.
- —пособствует синтезу коллагеновых и эластиновых волокон.
- ѕовышает упругость и тонус кожи.
- ѕридает коже здоровый красивый цвет.
- –азглаживает мелкие морщинки.
- ѕомогает отслаиванию ороговевших чешуек поверхностного сло€ кожи.
- ѕомогает сн€ть различные кожные воспалени€, что особенно хорошо при проблемной и чувствительной коже.
2) 5 мл. масло жожоба (1 ч.л.)
- ћасло жожоба - жидкий воск, уникальное масло, по свойствам аналогичное спермацету, не имеет себе равных по химическому составу в растительном мире. —войства масла жожоба обусловлены вход€щими в его состав аминокислотами - протеином, который напоминает коллаген - воскообразное вещество.
- ќмолаживает, регенерирует, сохран€ет естественную влажность кожи. ќбладает противовоспалительным и нормализующими свойствами, поэтому полезно как дл€ сухой, так и дл€ жирной кожи.
- ѕитает, увлажн€ет, глубоко проникает в поры, идеально ухажива€ за кожей лица, шеи, декольте.
- —мешива€сь с ингредиентами кожного сала на поверхности кожи, масло жожоба образует газопроницаемый защитный слой.
3) 5 мл. масла грецкого ореха (1 ч.л.)
- ”меньшает морщины и сухость кожи, избавл€ет от небольших трещин.
- –егенерирующее, омолаживающее, тонизирующее, увлажн€ющее средство.
стати, если принимать масло грецкого ореха внутрь по 1 ложке в день, можно похудеть.
Ёфирные масла (начальна€ дозировка эфирных масел- 0,5-1% (1к. на 5 мл. основного масла):
4) 1 капл€ сантала
- ѕротивовоспалительное средство..
- —нимают раздражение.
- Ћечит акне (угрева€ сыпь).
- ѕомогает избавитьс€ от токсинов.
5) 1 капл€ розового дерева
- ќбладает антибактериальным и тонизирующим действием.
- ѕитает, увлажн€ет и нормализует обменные процессы в коже.
- –азглаживает мелкие морщинки, выравнивает тон лица и сглаживает сосудистый рисунок
- ”дал€ет пигментные п€тна и мелкие рубцы
- «ащищает кожу от неблагопри€тных факторов среды и защищает от солнца.
6) 1 капл€ чайного дерева
- »меет около 40 биологически активных веществ, которые обуславливают его способность убивать микроорганизмы (грибы, вирусы, бактерии).
- ќказывает регенерирующее действие на кожу.
¬итамины:
7) 2 капсулы витамина ≈
- јнтиоксидант, который защищает клетки от патологического перекисного окислени€, которое приводит к их старению и гибели.
- ”лучшает питание кожи.
ѕроцесс приготовлени€:
- ¬ключаем плиту и ставим на нее кастрюльку с водой. огда закипит вода, выключаем плитку и ставим в неЄ баночку дл€ крема с предварительно смешанными базовыми маслами. ѕрогреваем масла до температуры не выше 70 градусов, иначе они могут потер€ть р€д своих свойств.
- ќтдельно смешиваем эфирные масла, и когда смесь базовых масел остынет, добавл€ем в них эфиры и витамин ≈. «акрываем крышкой и ставим в холодное место, храним там же.
ƒанным средством следует пользоватьс€ каждый день в течение трех недель, а затем на неделю сделать перерыв. ќ каких-то видимых результатах можно судить через 4 недели – именно столько длитс€ процесс обновлени€ клеток. Ќаносить лучше вечером на лицо, шею и область декольте, легко массиру€ по массажным лини€м. ѕосле массажа маску-крем оставить на лице на 15 минут, после чего не смывать, а слегка пропитать салфеткой.
P.S.: –езультаты будут очень даже замечательные не только у тех, у кого морщинки только наметились, но и у кого они уже довольно глубокие.
јркалика
|
Ќесколько статей по лечению содой |
ƒневник |
—ода-универсальное средство по своей доступности в применении как в пищевом рационе, так и в медицине. Ќо ее изумительные предохранительные и целительные свойства еще недостаточно осознаны и не столь широко примен€ютс€. ј ведь гидрокарбонат натри€ (или сода) входит, как главный инградиент, в состав нашей крови. (ќб этом указывала ≈.».–ерих). —ода входит в состав плазмы крови, а также лимфоплазмы, в которой наход€тс€ лимфоциты. ¬озможно, сода энергетически питает лимфоциты - клетки, ответственные за иммунный ответ организма.
—реди людей бытует превратное мнение,иногда поддерживаемое и медиками, что длительный и частый прием соды оказывает отрицательное воздействие на работу слизистой оболочки желудка. ј прием ее люд€м с пониженной кислотообразующей функцией желудка или анацидном состо€нием его противопоказан, что неверно. Ёто доказано лабораторными исследовани€ми на кафедре физиологии человека и животных в √омельском госуниверситете в 1982 г.; о вли€нии соды на кислотообразование, на работу слизистой желудка (на собаках с фистулами желудка). ќпытным путем подтверждено, что питьева€ сода, облада€ кислотонейтрализующим действием, не оказывает ни возбуждающего, ни тормоз€щего вли€ни€ на кислотовыделительную функцию желудка (журнал "«дравоохранение Ѕелоруссии" N1 ; 1982 г.) —ледовательно, прием соды может быть рекомендован при любом состо€нии кислотности желудка, в т.ч. при гастрите с пониженной кислотностью.
«а последние 20 лет в ќтечественной медицине были интересные разработки по применению соды при различных заболевани€х и состо€ни€х организма.
—ода с химической точки зрени€ представл€ет собой соединение катиона натри€ и аниона гидрокарбоната, которое при введении в организм активно включаетс€ в коррекцию кислото-щелочного равновеси€. ѕри многих т€желых заболевани€х в клетках и ткан€х организма наблюдаетс€ ацидоз (или закисление организма), недостаток катионов кали€ и избыток натри€, что приводит к подавлению энергетических биохимических обменных процессов в клетках (тормозитс€ цикл ребса), снижению усвоени€ кислорода, уменьшению жизнеспособности как каждой клетки, так и всего организма. ѕоложительное оздоравливающее действие соды уникально. Ѕлагодар€ введению анионов угольной кислоты (Ќ—ќ ) повышаетс€ щелочной резерв организма: анион угольной кислоты выводит через почки избыток анионов хлора и натри€, что приводит к уменьшению отеков, снижению повышенного давлени€, в результате чего повышаетс€ валентность тканевых буферных систем, что создает условие дл€ вхождени€ катиона кали€ в клетки, и тем самым объ€сн€етс€ калийсберегающий эффект соды.
¬ результате в клетках восстанавливаютс€ и повышаютс€ биохимические и энергетические процессы,увеличиваетс€ гемодинамика и усвоение кислорода ткан€ми, что приводит к улучшению самочувстви€ и трудоспособности.
этим выводам пришли медики на кафедре терапии центрального института усовершенствовани€ врачей в ћоскве (я.ѕ.÷аленчук,√.ѕ.Ўульцев и др. ∆урнал "“ерапевтический архив" N7 1976 г, N7 1978 г), которые изучали применение гидрокарбоната натри€ при хроническом гломерулонефрите, пиелонефрите, хронической почечной недостаточности внутривенным и ректальным способами, что вызвало изменение в состо€нии здоровь€ больных, увеличением кислотовыделительной функции почек, увеличением клубочковой фильтрации, снижением артериального давлени€, уменьшением остаточного азота в крови, уменьшением отеков.
ѕри т€желом шоке получены хорошие результаты лечени€ внутриартериальным введением раствора соды. ¬ нашей практике наблюдалс€ случай быстрого и эффективного купировани€ отека легких у больной с т€желым течением инфаркта миокарда после внутривенного введени€ 4% р-ра соды 200,0. Ѕольна€ в удовлетворительном состо€нии была переведена из реанимации в общую палату.
ќтмечено положительное действие соды при болезн€х движени€, или морской болезни. √идрокарбонат натри€ повышает устойчивость вестибул€рного анализатора к действию угловых ускорений,угнета€ вращательный и послевращательный нистагм (ј.ћ.—утов,».–.¬еселов," осмическа€ медицина и авиокосмическа€ медицина" N3 1978г).
Ёффективность комплексного применени€ соды и хлорида кали€ при лечении морской болезни отмечена ».».ѕотаповым,ј.√. узнецовым ("¬естник оториноларингологии" N3 1977г). ѕод наблюдением были 6 членов экипажа судна, у которых укачивание про€вл€лось в многократной рвоте, тошноте, снижении артериального давлени€, резком снижении работоспособности во врем€ шторма. ѕосле введени€ р-ра —l и NаЌ—ќ рвота прекратилась, тошнота уменьшилась, восстановилась работоспособность.
јвторы статьи утверждают, что положительный эффект обусловлен повышением потреблени€ кислорода ткан€ми, нормализацией де€тельности сердечно-сосудистой системы, повышением натрий- и хлоруреза. ”становлено, что гидрокарбонат натри€ обладает четким калийсберегающим свойством.
ј потер€ ионов кали€ отмечаетс€: при различных стади€х гипертонической болезни, сердечно-сосудистых заболевани€х, в первые дни после полостных операций, при перитоните, остром панкреатите, диабете, хроническом пиелонефрите и гломерулонефрите, почечной недостаточности, различных нарушени€х и болезн€х вестибул€рного аппарата и морской болезни. —ледовательно, сода должна примен€тьс€ шире в комплексном лечении этих заболеваний.
ћедики рыма советуют при отравлении хлорофосом и ‘ќ— нар€ду с введением антидотов - атропина и дипироксима примен€ть соду и глюкозу, что приводит к значительному улучшению мозгового кровообращени€, увеличению напр€жени€ и поглощени€ кислорода клетками мозга. “акже сода увеличивает диффузию кислорода и углекислого газа через альвеол€рные мембраны легких,ослабл€ет ацидоз.
ё.Ќ.–ерих в своем труде "ѕо тропам —рединной јзии" описывает случай острого отравлени€ лошадей неизвестной травой. ¬ “ибете, при употреблении ее животные погибали. ¬ этом случае удалось сохранить жизнь животным, напоив их содовым раствором.
Ќами проведено наблюдение за показани€ми общего и биохимического анализа крови после трехмес€чного приема соды с валерианом. ѕримечательно увеличение как общего количества лейкоцитов (белых клеток крови,имеющими непосредственную св€зь с тонким телом) на 1,4 10 /л, так и непосредственно лимфоцитов, отвечающим за состо€ние клеточного иммунитета, на 37%. Ѕиохимический анализ показал возрастание электролитов (до приема соды показатели были несколько снижены), повышение уровн€ белка до верхних границ нормы (при отсутствии в пищевом рационе м€сных и рыбных продуктов в течении 7 лет). ќпыт продолжаетс€ дл€ более углубленного изучени€.
ƒоктора из ¬еликобритании считают, что нашли доступное и дешевое средство, которое может значительно облегчить страдани€ пациентов с почечной недостаточностью. Ёто чудесное лекарство – обыкновенна€ пищева€ сода. –абота почек ухудшаетс€ гораздо медленнее, если пациент принимает немного соды раз в день. –езультаты исследовани€ были опубликованы в журнале Journal of the American Society of Nephrology.
аждый год около 400 британцев умирают от почечной недостаточности, не дождавшись донорского органа, а еще 3 миллиона страдают хроническими заболевани€ми почек, которые могут привести к необходимости диализа или трансплантации. ќбычна€ пищева€ сода, котора€ есть практически у всех, может помочь многим люд€м, страдающим почечной недостаточность. ≈е употребление заметно замедл€ет прогрессирование болезни.
ƒоктора давно интересовались, каким образом использование в лечении пищевой соды может повли€ть на почки пациентов, страдающих от низкого уровн€ бикарбоната. Ёто состо€ние называют метаболическим ацидозом. »сследователи из оролевского √оспитал€ в Ћондоне привлекли к своей работе 134 пациента, дава€ им небольшую дозу соды каждый день нар€ду с обычными лекарствами. „ерез год работа их почек ухудшилась в гораздо меньшей степени, чем у тех больных, которых не лечили содой. ”ровень ухудшени€ работы их почек был не намного заметнее, чем при обычном процессе старени€. ќни также реже страдали от последней стадии почечной болезни, требующей диализа.
«“акое простое средство, как пищева€ сода, если его правильно использовать, может быть чрезвычайно эффективным. ќно очень полезно дл€ людей с почечной недостаточностью. ќднако доза соды должна тщательно подбиратьс€, и такое лечение следует проводить под надзором специалистов», - заключил руководитель исследовани€ профессор ренальной медицины из оролевского √оспитал€ в Ћондоне ћагди якуб.
»сточник

ƒобавлено через 27 минут
Ќашел ссылку на оригинальные материалы упоминаемых исследований, где в р€ду прочего даютс€ и конкретные рекомендации больным с почечной недостаточностью по самосто€тельному применению соды. сожалению текст публикации на английском.
http://jasn.asnjournals.org/content/12/11/2418.full
” человека показатель кислотности pH крови должен находитьс€ в норме в пределах 7,35-7,47. ≈сли pH меньше 6,8 (очень кисла€ кровь, сильнейший ацидоз), то наступает смерть организма (Ѕ—Ё, т.12, с. 200).
¬ насто€щее врем€ большинство людей страдает от повышенной кислотности организма (ацидоза), име€ pH крови ниже 7,35. ѕри pH меньше 7,25 (сильный ацидоз) должна назначатьс€ ощелачивающа€ терапи€: прием соды от 5 г до 40 г в сутки (—правочник терапевта, 1973, с. 450, 746). ѕри отравлении метанолом внутривенна€ суточна€ доза соды достигает 100 г (—правочник терапевта, 1969, с. 468.). ѕричинами ацидоза €вл€ютс€ €ды в пище, воде и воздухе, лекарства, пестициды. Ѕольшое самоотравление людей психическими €дами происходит от страха, беспокойства, раздражени€, недовольства, зависти, злобы, ненависти, которые сейчас очень усилены благодар€ нарастающим волнам осмического ќгн€. ѕри потере психической энергии почки не могут удерживать в крови высокую концентрацию соды, котора€ при этом тер€етс€ вместе с мочой. Ёто друга€ причина ацидоза: потер€ психической энергии ведет к потере щелочей (соды). ƒл€ коррекции ацидоза назначают 3-5 г соды в сутки (ћашковский ћ.ƒ. Ћекарственные средства, 1985, т.2, с. 113).
—ода, уничтожа€ ацидоз, повышает щелочные резервы организма, сдвигает кислотно-щелочное равновесие в щелочную сторону (pH примерно 1,45 и выше). ¬ щелочном организме происходит активаци€ воды, т.е. диссоциаци€ ее на ионы Ќ+ и OH- за счет аминных щелочей, аминокислот, белков, ферментов, нуклеотидов –Ќ и ƒЌ . ¬ активированной воде, насыщенной огненной энергией организма, улучшаютс€ все биохимические процессы: ускор€етс€ синтез белка, быстрее обезвреживаютс€ €ды, активнее работают ферменты и аминные витамины, лучше действуют аминные лекарства, имеющие огненную природу и биологически активные вещества.
«доровый организм дл€ пищеварени€ вырабатывает сильно щелочные пищеварительные соки. ѕищеварение в двенадцатиперстной кишке происходит в щелочной среде под действием соков: панкреатический сок, желчь, сок бруттнеровой железы и сок слизистой оболочки двенадцатиперстной кишки. ¬се соки имеют высокую щелочность (ЅћЁ, изд. 2,т. 24, с. 634). ѕанкреатический сок имеет pH=7,8-9,0. ‘ерменты панкреатического сока действуют только в щелочной среде. ∆елчь в норме имеет щелочную реакцию pH=7,50-8,50. —екрет толстого кишечника имеет сильно щелочную среду pH=8,9-9,0 (ЅћЁ, изд. 2, т. 12, ст. ислотно-щелочное равновесие, с. 857). ѕри сильном ацидозе желчь становитс€ кислой pH=6,6-6,9 вместо нормы pH=7,5-8,5. Ёто ухудшает пищеварение, что приводит к отравлению организма продуктами плохого пищеварени€, образованию камней в печени, желчном пузыре, кишечнике и почках. ¬ кислой среде спокойно живут глисты опистархоза, острицы, аскариды, цепни и др. ¬ щелочной среде они гибнут. ¬ кислом организме слюна кисла€ pH=5,7-6,7, что приводит к медленному разрушению эмали зубов. ¬ щелочном организме слюна щелочна€: pH=7,2-7,9 (—правочник терапевта, 1969, с. 753) и зубы не разрушаютс€. ƒл€ лечени€ кариеса кроме фтора необходим прием соды дважды в день (чтобы слюна стала щелочной).
—ода, нейтрализу€ избыточные кислоты, повышает щелочные резервы организма, делает мочу щелочной, что облегчает работу почек (сберегает психическую энергию), сберегает глутаминовую аминокислоту, предотвращает отложение камней в почках.
«амечательным свойством соды €вл€етс€ то, что избыток еЄ легко выводитс€ почками, дава€ щелочную реакцию мочи (ЅћЁ, изд. 2, т. 12, с. 861). “Ќо следует приучать тело к ней длительно” (ћ.ќ., ч. 1, с. 461), т.к. защелачивание организма содой приводит к выведению большого количества €дов (шлаков), накопленных организмом за многие годы кислой жизни.
¬ щелочной среде с активированной водой многократно возрастает биохимическа€ активность аминных витаминов: ¬1 (тиамин, кокарбоксилаза), ¬4 (холин), ¬5 или –– (никотиномид), ¬6 (пиридоксаль), ¬12 (кобимамид). ¬итамины, имеющие огненную природу (ћ.ќ., ч. 1, 205) могут полностью про€вл€ть еЄ только в щелочной среде.
¬ кислой среде отравленного организма “даже лучшие растительные витамины не могут вы€вить своих лучших качеств (Ѕр., 13). “ћускус и гор€чее молоко с содой будут хорошим предохранителем. Ќасколько холодное молоко не соедин€етс€ с ткан€ми, настолько же гор€чее с содой проникает в центры” (ћ.ќ., ч. 1, п. 58.). ѕоэтому дл€ улучшени€ всасывани€ соды из кишечника еЄ принимают с гор€чим молоком. ¬ кишечнике сода реагирует с аминокислотами молока, образу€ щелочные натриевые соли аминокислот, которые легче, чем сода всасываютс€ в кровь, повыша€ щелочные резервы организма. Ѕольшие дозы соды с водой не всасываютс€ и вызывают понос, используютс€ как слабительное.
ƒл€ борьбы с аскаридами и острицами примен€ют аминную щЄлочь пиперазин, дополн€€ его клизмами соды (ћашковский ћ.ƒ., т. 2, с. 366-367). —ода примен€етс€ при отравлении метанолом, этиловым спиртом, формальдегидом, карбофосом, хлорофосом, белым фосфором, фосфином, фтором, йодом, ртутью и свинцом (—правочник терапевта, 1969).
–аствор соды, едкого натра и аммиака примен€ют дл€ уничтожени€ (дегазации) боевых отравл€ющих веществ ( ’Ё, т. 1, с. 1035). ƒл€ отвыкани€ от курени€: полоскание рта густым раствором соды или обмазывание полости рта содой со слюной: сода кладЄтс€ на €зык, раствор€етс€ в слюне и вызывает отвращение к табаку при курении. ƒозы малые, чтобы не нарушать пищеварени€.
—традающим морской болезнью производитс€ 3—5-кратное внутривенное капельное введение 200 мл 1 % раствора али€ гидрокарбоната или 200 мл раствора, содержащее кали€ хлорид и натри€ гидрокарбонат, с 3-дневным интервалом между инъекци€ми.
–ектальный способ лечени€ и профилактики средней и легкой формы морской болезни по своей эффективности не уступает внутривенным введени€м, которые предпочтительнее при лечении больных т€желой формой морской болезни. Ќесомненными достоинствами ректального введени€ натри€ гидрокарбоната €вл€етс€ его техническа€ простота. ”казанна€ методика не требует участи€ медицинского персонала, в большинстве случаев лечение можно назначать амбулаторно и проводить на дому, что в конечном итоге дает выраженный экономический эффект. Ќар€ду с этим не вызывают ударных перегрузок на почки и другие системы регул€ции кислотно-щелочного равновеси€, которые в определенной степени выражены сразу же при внутривенном введении натри€ гидрокарбоната и кали€ гидрокарбоната. ѕри ректальном введении натри€ гидрокарбоната вещество поступает через кровь во вне- и внутриклеточную жидкости постепенно и в меньшем количестве, чем при внутривенном введении, а почки не выдел€ют избытка натри€ гидрокарбоната, который возникает в крови во врем€ внутривенного введени€ вещества и в первые часы после него. ѕри ректальном введении повышение щелочного резерва организма не сопровождаетс€ резкими сдвигами в регул€ции кислотно-щелочного равновеси€ и способствует нормальному течению обменных процессов. Ќатри€ гидрокарбонат при ректальном введении минует печеночный барьер, поэтому он не подвергаетс€ метаболическим превращени€м.
ѕитьева€ сода повышает качество игры в теннисе.

”потребление в пищу гидрокарбоната натри€ (питьева€ сода) утром, в день матча позвол€ет теннисистам сохран€ть конкурентное преимущество. –андомизированное контролированное испытание, опубликованное в ∆урнале международного общества спортивного питани€ (Journal of the International Society of Sports Nutrition), показало, что спортсмены, принимавшие эту добавку, сохран€ли стабильность в профессиональной теннисной игре после проведение симулированного матча.
„ен- анг „анг из Ќационального “айваньского колледжа физической культуры провел это исследование совместно с командой других ученых. «ћы обнаружили, что добавка в виде гидрокарбоната натри€ может предотвратить ухудшение качества игры теннисиста, вызванное утомлением, которое часто можно увидеть во врем€ матчей. —табильность подачи и удара справа с отскока сохран€лась после симулированного мачта в группе участников, принимавших питьевую соду. — другой стороны, эффективность удара ухудшалась после мачта в группе, получившей плацебо».
ƒев€ть игроков в исследовании были произвольно выбраны дл€ приема напитка, содержащего гидрокарбонат натри€ или плацебо. «атем они прин€ли участие в квалификации до и после проведени€ симулированного матча. ѕроверка квалификации оценивала точность и стабильность подачи и ударов с отскока справа и слева, на обе стороны корта. омментиру€ результаты исследовани€, „анг сказал: «Ќасколько нам известно, это первое исследование, которое показало вли€ние питьевой соды на качество игры в теннисе. ¬ будущих исследовани€х необходимо изучить вли€ние бикарбоната на другие теннисные навыки, такие как удар с лета, укороченный удар, и измерить быстроту реакции и скорость бега.
»сточник 
—ами исследовани€:
Wu CL, Shih MC, Yang CC, Huang MH, Chang CK. Sodium bicarbonate supplementation prevents skilled tennis performance decline after a simulated match. J Int Soc Sports Nutr. 2010, vol.7, є1, pp.33.
"—одовый допинг" дл€ спортсмена.
Ѕританские исследователи из ”ниверситета в Ћоборо, задавшись вопросом, почему употребление соды перед спортивными соревновани€ми улучшает результаты, пришли к интересным выводам.
ѕосле употреблени€ соды некоторые плавцы улучшили свои результаты в среднем на 1,5 секунды в заплыве на 200 метров. ќказываетс€, пищева€ сода понижает кислотность желудка, и, следовательно, снижает количество побочных продуктов пищеварени€, таких как молочна€ кислота. »менно избыток молочной кислоты ведЄт к мышечным бол€м и быстрой утомл€емости при высоких нагрузках.
ќднако "содовый допинг" не может систематически использоватьс€ дл€ улучшени€ результатов, так как у многих спортсменов сода приводит к расстройству желудка.
‘армакологическое обеспечение и коррекци€ физической работоспособности в спортивной тренировке.
јвтор: ¬.Ћ.—мульский
Ћекци€ дл€ слушателей факультета повышени€ квалификации преподавателей физического воспитани€ вузов и факультета повышени€ квалификации руковод€щих физкультурных работников и тренеров / ¬.Ћ.—мульский. - иев: √»‘ , 1988. -19 с.
¬есьма существенной в плане положительного вли€ни€ на физическую работоспособность и протекание восстановительных процессов €вл€етс€ коррекци€, осуществл€ема€ с помощью таких биологически активных металлов, как Fe , Cu, Ni, Zn, Co, Mn, Mg. ѕоказано также, что использование этих микро- и макроэлементов на фоне витаминизации способствует сохранению нормальной иммунологической реактивности при напр€женных физических нагрузках.
этой группе препаратов относ€тс€ также средства, нейтрализующие продукты декомпенсированного метаболического ацидоза (сода и другие ощелачивающие препараты). ¬ качестве примера их использовани€ можно привести предложенный в 1931 г. ’.ƒеннигом следующий рецепт: лимоннокислый натрий - 5 г, бикарбонат натри€ - 3,5 г, лимоннокислый калий - 1,5 г. ѕредлагаема€ дневна€ доза рекомендовалась дл€ приема после еды в течение, двух дней до соревнований и двух дней после них. Ќа кафедре биохимии √»‘ изучена возможность коррекции развивающегос€ при мышечной де€тельности ацидоза, изменени€ обмена биорегул€торов и некоторых других процессов с помощью препарата "сложна€ солева€ смесь", созданного в »нституте биохимии имени ј.¬.ѕалладина јЌ ”——– под руководством академика јЌ ”——– ћ.‘.√улого. ќказалось, что этот препарат способствует повышению щелочных резервов и содержани€ биорегул€торов, что про€вл€етс€ в нетренированном и тренированном организме (ћ.». алинский и сотр.,1986). Ёто позвол€ет считать перспективным использование препарата в спортивной практике.
|
Ѕез заголовка |
Ёто цитата сообщени€ Angels_cat [ѕрочитать целиком + ¬ свой цитатник или сообщество!]
секретный крем моей мамы "Ќам года - не беда!"

†” моей мамы очень хороша€ кожа. ћорщины, под глазами, несмотр€ на возраст, слегка различимы. ≈е секрет прост - много лет вечерами она протирает веки камфорным маслом.
Ќо € рекомендую всЄ же пользоватьс€ не чистым камфорным маслом, а кремом на его основе.
ѕриготовьте нежный домашний камфорный крем специально дл€ кожи вокруг глаз. ¬озьмите 50 мл растопленного на вод€ной бане несоленого жира (нутр€ного свиного) и 50 мл камфорного масла, смешайте и перелейте в баночку. ƒва раза в неделю смазывайте этим кремом кожу перед сном.
|
Ѕез заголовка |
Ёто цитата сообщени€ Ћюдмила_ёрина [ѕрочитать целиком + ¬ свой цитатник или сообщество!]
 ƒумаете, приготовление крема в домашних услови€х это из области фантастики? „то составл€ющие не достать, а если и возможно достать — то дорого? Ќа примере этого крема мы покажем, что ¬ы заблуждаетесь
ƒумаете, приготовление крема в домашних услови€х это из области фантастики? „то составл€ющие не достать, а если и возможно достать — то дорого? Ќа примере этого крема мы покажем, что ¬ы заблуждаетесь ![]() »ме€ час свободного времени, вполне возможно приготовить самосто€тельно лЄгкий увлажн€ющий крем, а все необходимые ингредиенты дл€ него можно купить в соседней аптеке. «ачем все это, — спросите вы. ¬се очень просто. ¬о-первых, кремоварение — это творчество, интересное и увлекательное хобби. ¬о вторых, кто кроме ¬ас знает, что нужно ¬ашей коже? огда ¬ы сделаете крем самосто€тельно, ¬ы будете точно знать, что и в каком количестве ¬ы туда положили. акого качества использованные составл€ющие — т.е. получите именно то, что нужно именно ¬јћ!
»ме€ час свободного времени, вполне возможно приготовить самосто€тельно лЄгкий увлажн€ющий крем, а все необходимые ингредиенты дл€ него можно купить в соседней аптеке. «ачем все это, — спросите вы. ¬се очень просто. ¬о-первых, кремоварение — это творчество, интересное и увлекательное хобби. ¬о вторых, кто кроме ¬ас знает, что нужно ¬ашей коже? огда ¬ы сделаете крем самосто€тельно, ¬ы будете точно знать, что и в каком количестве ¬ы туда положили. акого качества использованные составл€ющие — т.е. получите именно то, что нужно именно ¬јћ!
≈сли € убедила ¬ас. или хот€ бы капельку, но заинтересовала, прочтите наш мастер-класс по приготовлению крема в домашних услови€х ![]() ¬се вопросы и пожелани€ можно оставл€ть в комментари€х.
¬се вопросы и пожелани€ можно оставл€ть в комментари€х.
ƒл€ приготовлени€ увлажн€ющего крема берем (все куплено в аптеке):
1,5 чайной ложки лецитина;
6 чайных ложек дистиллированной воды;
2 чайные ложки касторового масла;
1,5 мл глицерина;
2 мл сока алоэ;
2 мл настойки прополиса.
Ќам понадоб€тс€ следующие инструменты и посуда:
‘а€нсова€ или фарфорова€ емкость дл€ приготовлени€ (Ємкости и инструменты желательно простерилизовать на водной бане, либо протереть спиртом);
ќдноразовый шприц на 5 мл (отмер€ть ингредиенты);
—текл€нна€ или дерев€нна€ палочка дл€ перемешивани€;
ћиксер (желательно с плоской насадкой дл€ взбивани€ крема, но можно и обычным венчиком, взбивать на самой маленькой скорости).
ѕриготовление.
ќтмерьте 1,5 чайные ложки лецитина
«амочите лецитин в воде дл€ того, что бы он набух — желательно оставить посто€ть на час, что бы лецитин лучше разошелс€. ≈ще можно подогреть воду до 40 градусов на водной бане-тогда лецитин разойдетс€ без комков. √лавное не перегреть воду!
„ерез час аккуратно перемешайте лецитин и добавьте:
асторовое масло
√лицерин
—ок алоэ
Ќастойку прополиса
Ќесколько слов об ингредиентах дл€ крема. √лицерин будет отвечать за увлажнение, алоэ – за питание и поддержание кожи в тонусе, лецитин-мощный питательный компонент а в нашем случае это еще и эмульгатор, ну а прополис — эффективный противоспалительный ингредиент, кроме того, он будет служить легким консервантом.
¬збейте полученную смесь миксером — причем начинайте на маленькой скорости и постепенно переходите на большую. ¬рем€ взбивани€ – от 3 до 5 минут. —начала крем начнет густеть и цепл€тьс€ за миксер – а потом станет похож на промышленный крем и перестанет т€нутьс€. √лавное не перевзбивать — иначе наш крем превратитс€ в молочко!
ѕоследний, и самый при€тный этап-переложить крем в баночку и попробовать на себе. ” нас получилс€ замечательный легкий увлажн€ющий крем, идеально подход€щий дл€ всех типов кожи. ќн легко впитываетс€ и не оставл€ет пленки, а главное-полностью натурален.
сожалению, из-за отсутствие нормального консерванта он может хранитс€ в холодильнике около 2 недель — не больше.
|
Ѕез заголовка |
ƒневник |

„то такое крем
Ѕольшинство из нас посто€нно пользуютс€ кремами, не задумыва€сь о том, что же это такое и почему крем приносит нашим лицам (и телам) пользу (реже - вред).
„то вообще такое - крем?
ѕо сути крем - это эмульси€, котора€ образуетс€ при смешивании жира (масла) и воды. ∆ир с водой просто так не смешиваютс€. ѕоэтому, чтобы получить текстуту эмульсии, дл€ производства крема нужен эмульгатор.
—обственно, масло + эмульгатор + вода = крем.
ѕо какой-то чудесной причине вы решили впервые сделать крем самосто€тельно.
–ецепты, найденные в сети, типа "натрите на терке банан, смешайте его с творогом, добавьте меда и сливок, перемешайте все и выбейте в полученную кремообразную массу куриный желток" почему-то вас не устраивают.
я даже догадываюсь, почему. “о, что в интернете "одна баба рассказала", описыва€ подобные рецепты "из бабушкиного сундучка", на самом деле кремами не €вл€етс€. Ёто маски, причем одноразовые, потому что они очень скоропорт€щиес€.
рем же - это крем. Ќатуральный крем, сваренный дома, по консистенции, как правило, точно такой же, как и магазинный, только лучший по составу. 
¬ообще, кремоварение существовало давным-давно. –ассказывают даже, что на раскопках ƒревнего ≈гипта находили чудом выжившие и дошедшие до наших дней емкости с кремом, причем, состав их не сильно отличаетс€ от современных. ¬ерить в это или нет - решать вам. я ни разу не участвовала в раскопках ƒревнего ≈гипта. опала только ’ерсонес. ремов там не находила, однако? € полагаю, что такое вполне возможно. ¬едь даже в глубокой древности были масла, вода, мед, воск, соль, растени€, а значит, и выт€жки из них, огонь, баночки и палочки дл€ перемешивани€ - все, что нужно дл€ производства хорошего крема.
ј вот рецепты вековой-полуторавековой давности мне известны. ¬ XVIII-XIX-XX веках крем варили вовсю.
»так. „тобы приготовить крем, нам понадоб€тс€ масло, эмульгатор, вода неглубокий ковш и 2-3 стекл€нных баночки (можно вз€ть баночки от детского питани€).†
|
Ѕез заголовка |
Ёто цитата сообщени€ Sofa54 [ѕрочитать целиком + ¬ свой цитатник или сообщество!]
ремоварение в домашних услови€х – рецепты крема дл€ сухой кожи
 ѕод воздействием солнечных лучей, ветра, холода, вследствие употреблени€ всевозможных средств бытовой химии дл€ дома наша кожа тер€ет драгоценную влагу и пересыхает. ≈сли воврем€ не прин€ть меры, могут по€витьс€ глубокие и болезненные трещины, которые достав€т крайне непри€тные ощущени€.
ѕод воздействием солнечных лучей, ветра, холода, вследствие употреблени€ всевозможных средств бытовой химии дл€ дома наша кожа тер€ет драгоценную влагу и пересыхает. ≈сли воврем€ не прин€ть меры, могут по€витьс€ глубокие и болезненные трещины, которые достав€т крайне непри€тные ощущени€.
я хочу поделитьс€ с вами рецептами кремов дл€ сухой кожи, которые можно приготовить в домашних услови€х.
рем дл€ сухой кожи на основе кокосового масла
35 гр. масла кокоса
2 ч. ложки масла ореха макадами€
10 гр. пчелиного воска
4 ч. ложки воды
1/2 ч. ложки буры
5 капель эфирного масла герани
5 капель эфирного масла иланг-иланг
ѕриготовление:
Ќа вод€ной бане разогреть масла и воск до полного растворени€ воска.
¬одную фазу довести до кипени€ и растворить в ней буру. ѕоместить емкость с раствором буры на вод€ную баню и подогревать в течение 15 минут периодически помешива€.
∆ирную фазу взбить миксером в пену, затем, продолжа€ взбивать, тоненькой струйкой влить раствор буры. ¬збивать, пока крем не загустеет, затем добавить эфирные масла, остудить и переложить крем в баночку.
рем-масло дл€ сухой и потрескавшейс€ кожи рук и ног
7 ч. ложек масла растопленного масла ши
2 ч. ложки масла жожоба
1 ч. ложка масла календулы
25 капель витамина ≈
15 капель эфирного масла лимона
8 капель эфирного масла пачули
9 капель эфирного масла лаванды
»нгредиенты перемешать, нанести на проблемные участки кожи и обмотать целлофановой пленкой.
—ерум дл€ сухой кожи лица
1 ч. ложка масла ореха макадами€
1 ч. ложка масла авокадо
1 ч. ложка масла кокоса
1 ч. ложка масла зародышей пшеницы
ѕо 2 капли эфирных масел герани, сандалового дерева, сосны, эвкалипта и лаванды.
¬се перемешать и наносить на кожу после умывани€ перед сном.
|
Ѕез заголовка |
Ёто цитата сообщени€ Angels_cat [ѕрочитать целиком + ¬ свой цитатник или сообщество!]

–ецепт N1. ћаска дл€ глаз из сырого картофел€: быстро очистить картофелину, пропустить через комбайн или натереть на терке, разделить на две части, завернуть в марлю и наложить на веки на 15-20 минут. «атем смыть прохладной водой, чаем или настоем ромашки. ≈сли не хочетс€ возитьс€ с теркой, можно просто нарезать картофель на круглые тонкие дольки и обложить ими веки.
–ецепт N2. Ќатуральна€ маска от морщинок под глазами из картофел€ и сливок:
тертый сырой картофель смешать со сливками, прилечь и нанести смесь на нижние веки. ј на верхние положить тампоны, смоченные крепким чаем. ”же через 15 минут глаза заблест€т, а кожа станет удивительно гладкой и молодой.
–ецепт N3. ћаска от син€ков под глазами из белого хлеба с молоком:
м€киш белого хлеба смешать с молоком, через 5 мин. нанести под глаза на 15 мин., делать через день.
–ецепт N4. Ќатуральна€ маска от морщин под глазами:
50 мл оливкового масла смешать с 10 мл масл€ного раствора витамина ≈ и каждый вечер вбивать смесь подушечками пальцев в кожу вокруг глаз в течение 5 мин.«атем тщательно удал€ть излишки масла бумажной салфеткой.
–ецепт N5. ћаска дл€ уставших глаз из виноградного сока:
полезно питать глаза виноградным соком. –азрезать виноградинку и провести по нижнему веку глаза. √лаз получает натуральное питание.
–ецепт N6. ƒомашн€€ маска из картошки и петрушки от морщин под глазами:
запарить половинку натЄртой вареной картофелины отваром петрушки и чуть капнуть растительного масла, выложить все это на кусочки марли и положить маску на глаза на пол часа перед сном.
–ецепт N7. “рав€ное масло дл€ век от морщин:
вз€ть по 1 ч. ложке сухих трав: лопух, календулу, м€ту. —месь трав залить 50-100 гр. оливкового масла. Ќастаивать травы на масле 7 дней в темном месте. ѕроцедить готовое трав€ное масло. —мазывать этим маслом веки за 2-3 часа до сна. ƒержать 5-10 мин., потом промокнуть салфеткой излишки масла.
–ецепт N8. “ибетска€ маска от морщин: приготовить крем - взбить 3 сырых белка с 15 гр. оливкового масла и столовой ложкой отвара лавровых листьев. ƒл€ этого 5 листиков залить 300 гр. кип€тка и на небольшом огне варить 10 минут. ƒобавить 10 гр. жженных квасцов (продаютс€ в аптеке). “щательно перемешать весь состав до получени€ однородной массы. »з куска фланели вырезать маску дл€ лица, оставив отверсти€ дл€ глаз, носа и рта. ѕриготовленным кремом пропитать подготовленную ткань. ѕоложить маску на лицо и оставить на ночь. ≈сли морщины расположены локально (например, "гусиные лапки" вокруг глаз), то можно сделать маску только на эти участки.
|